Synthesis of berberine derivatives and application of berberine derivatives in preparing anti-tumor drug and anti-tumor drug composition in combination with adriamycin
A technology of berberine and its derivatives, applied in the field of medicinal chemistry, can solve the problems of insignificant anti-tumor effect and toxic and side effects of doxorubicin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
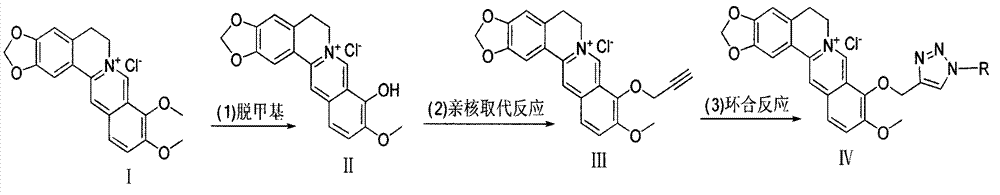
[0048] Embodiment 1, the synthesis of berberine chloride (II)
[0049] Weigh 8g (0.0216mol) of berberine chloride, put it into a 250ml flask, add DMF120ml, reflux in an oil bath at 190°C for 25-30min, TLC (dichloromethane:methanol=10:1) detects that the raw material point just disappears and the impurity point The reaction was stopped when it did not appear (product Rf value about 0.4, red). The solvent was evaporated to dryness to obtain 7 g of crude product BBR-1 with a yield of 85%, which could be directly used in the next reaction.
Embodiment 2
[0050] Example 2 Synthesis of 9-O-propynyl berberine (compound III)
[0051] Weigh 0.54g (1.67mmol) of berbererythrine chloride (compound II) into a 100ml flask, add 0.24g (2.03mmol) of propargyl bromide, 60ml of acetonitrile, and reflux at 70-80°C for 2.5h. TLC detection (dichloromethane:methanol=10:1), visible product spot (Rf value about 0.45, yellow), and product yellow precipitate appeared at the bottom of the flask. Column chromatography (dichloromethane:methanol=25:1) yielded 0.35 g of the product with a yield of 65%.
Embodiment 3
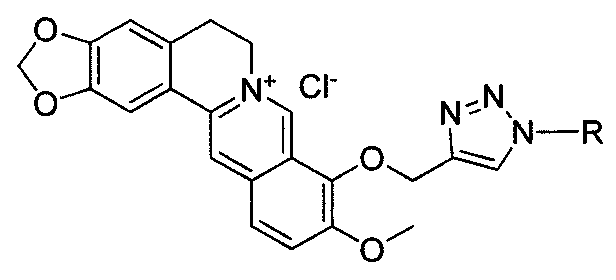
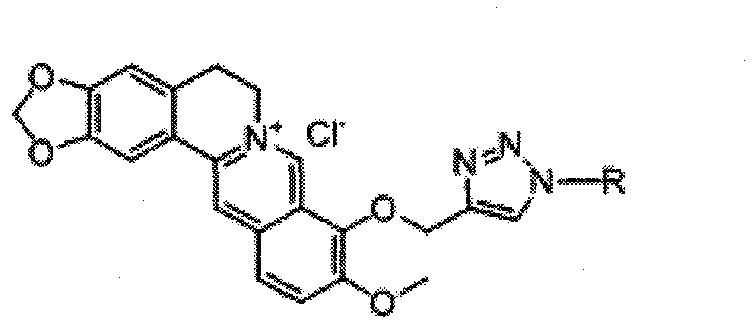
[0052] Example 3 Synthesis of compound 1-propyl-4-(9-O-methylene berberine)-1 hydrogen-1,2,3-triazole (compound 3 in Table 1)
[0053] Add 50mgNaN to the 50ml flask 3 (0.77mmol), 76mg1-bromo-n-propane (0.6mmol) 10ml DMF70 ℃ stirring 3h, cooling, put in 9-O-propynyl berberine (II) 160mg (0.4mmol), the CuSO 4 .5H 2 O30mg and sodium ascorbate 50mg were prepared into 5ml aqueous solutions respectively, and added to the reaction system. Continue to react for 3h, TCL detection (dichloromethane:methanol=10:1), the product point yellow (Rf value about 0.45). Add 10% ammonia water to the reaction solution, extract three times with 100ml of dichloromethane, wash the extract twice with 5ml of 12% hydrochloric acid, concentrate and separate by column chromatography (dichloromethane:methanol:triethylamine=25:1:trace amount) , to obtain 40mg of compound 3. 1 H NMR (300MHz, DMSO) δ9.72(s, 1H), 8.95(s, 1H), 8.40(s, 1H), 8.20(dd, J=9.2, 3.8Hz, 1H), 8.00(d, J= 9.1Hz, 1H), 7.78(d, J=3.3Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com