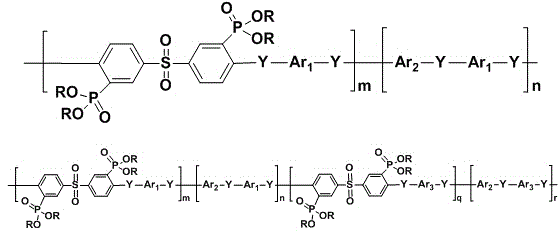
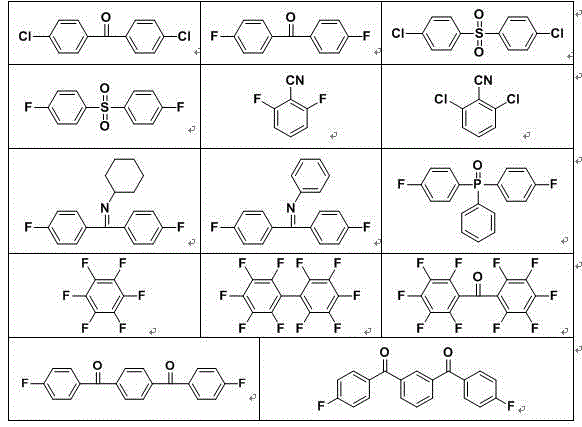
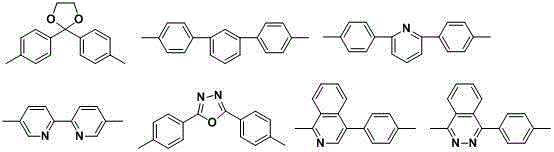
Poly(aromatic (thio)ether sulfone material containing phosphonate group and preparation method and application of poly(aromatic (sulfide)ether sulfone material
A phosphonate group and aromatic technology, which is applied in the field of new polymer materials, synthesis of phosphonylated polyarylether polymers and their preparation, can solve the problems of easy deformation, increased water treatment costs, and weak resistance And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 3,3 , -Bis(diethoxyphosphono)-4,4 , - Preparation of difluorodiphenyl sulfone:
[0039] (1) 3,3 , -Dibromo-4,4 , - Preparation of difluorodiphenyl sulfone:
[0040]
[0041] To a 250 mL three-necked bottle, add 4,4, -25.4 g (0.1 mol) of difluorodiphenyl sulfone, 150 mL concentrated sulfuric acid, mechanically stirred to dissolve, added 39.2 g (0.22 mol) of N-bromosuccinimide in batches, reacted at room temperature overnight, and poured the reaction solution into In ice water, a solid was precipitated, filtered, washed with water, and recrystallized from toluene to obtain 30 g of white crystals, with a yield of 73%. 1 H-NMR (DMSO- d 6 ): δ = 8.46-8.44 (m, 2 H), 8.15-8.11 (m, 2 H), 7.65 (t, J = 8.8 Hz, 2 H) ppm.
[0042] (2) 3,3 , -Bis(diethoxyphosphono)-4,4 , - Preparation of difluorodiphenyl sulfone:
[0043]
[0044] Will 3,3 , -Dibromo-4,4 , -Difluorodiphenyl sulfone and triethyl phosphite are fed according to the ratio of 1:8.0, N 2 Protected, th...
Embodiment 2
[0046] 3,3 , -Bis(diethoxyphosphono)-4,4 , - Preparation of dichlorodiphenyl sulfone:
[0047] (1) 3,3 , -Dibromo-4,4 , - Preparation of dichlorodiphenyl sulfone:
[0048]
[0049] To a 250 mL three-necked bottle, add 4,4 , -28.7 g (0.1 mol) of dichlorodiphenyl sulfone, 150 mL concentrated sulfuric acid, mechanically stirred to dissolve, added 39.2 g (0.22 mol) of N-bromosuccinimide in batches, reacted overnight at room temperature, poured the reaction solution into In ice water, a solid was precipitated, filtered, washed with water, and recrystallized from toluene to obtain 36 g of white crystals, with a yield of 81%. 1 H-NMR (DMSO- d 6 ): δ = 8.45-8.43 (m, 2 H), 8.06-8.04 (m, 2 H), 7.88 (t, J = 8.4 Hz, 2 H) ppm.
[0050] (2) 3,3 , -Bis(diethoxyphosphono)-4,4 , - Preparation of dichlorodiphenyl sulfone:
[0051]
[0052] Will 3,3 , -Dibromo-4,4 , -Dichlorodiphenyl sulfone and triethyl phosphite are charged according to the ratio of 1:8.0, N 2 Protected, ...
Embodiment 3
[0054] Preparation of phosphonate polymer (I-1):
[0055]
[0056] (Ⅰ-1)
[0057] Under nitrogen atmosphere, the 3,3 , -Bis(diethoxyphosphono)-4,4 , -difluorodiphenylsulfone (7.37 g, 14.0 mmol), 4,4 · -Dichlorodiphenylsulfone (1.72 g, 6.0 mmol), biphenol (3.72 g, 20.0 mmol), anhydrous potassium carbonate (3.18 g, 23.0 mmol), N,N-dimethylacetamide (DMAc, 56 mL), toluene (28 mL) were mixed and heated to 160°C, water was separated for 4 hours, the toluene was distilled off, the temperature was raised to 180°C, and the reaction was carried out for 36 hours. Slowly pour the reaction solution into 400 mL of deionized water to obtain a white strip polymer, change the water, keep the temperature at 80°C, boil in water three times, each time for 6 hours, dry, and then vacuum dry at 100°C for 24 hours to obtain white fibers The obtained polymer was 11.22 g, the yield was 95%, and the intrinsic viscosity was 0.54 dL / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com