Substituted diaryl ester compound, and preparation method and application thereof
A technology of compound and diaryl ether, which is applied in the field of substituted diaryl ether compounds and their preparation, can solve problems such as severe adverse reactions of myocardial infarction, achieve the effect of inhibiting malignant proliferation phenotype and promoting expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
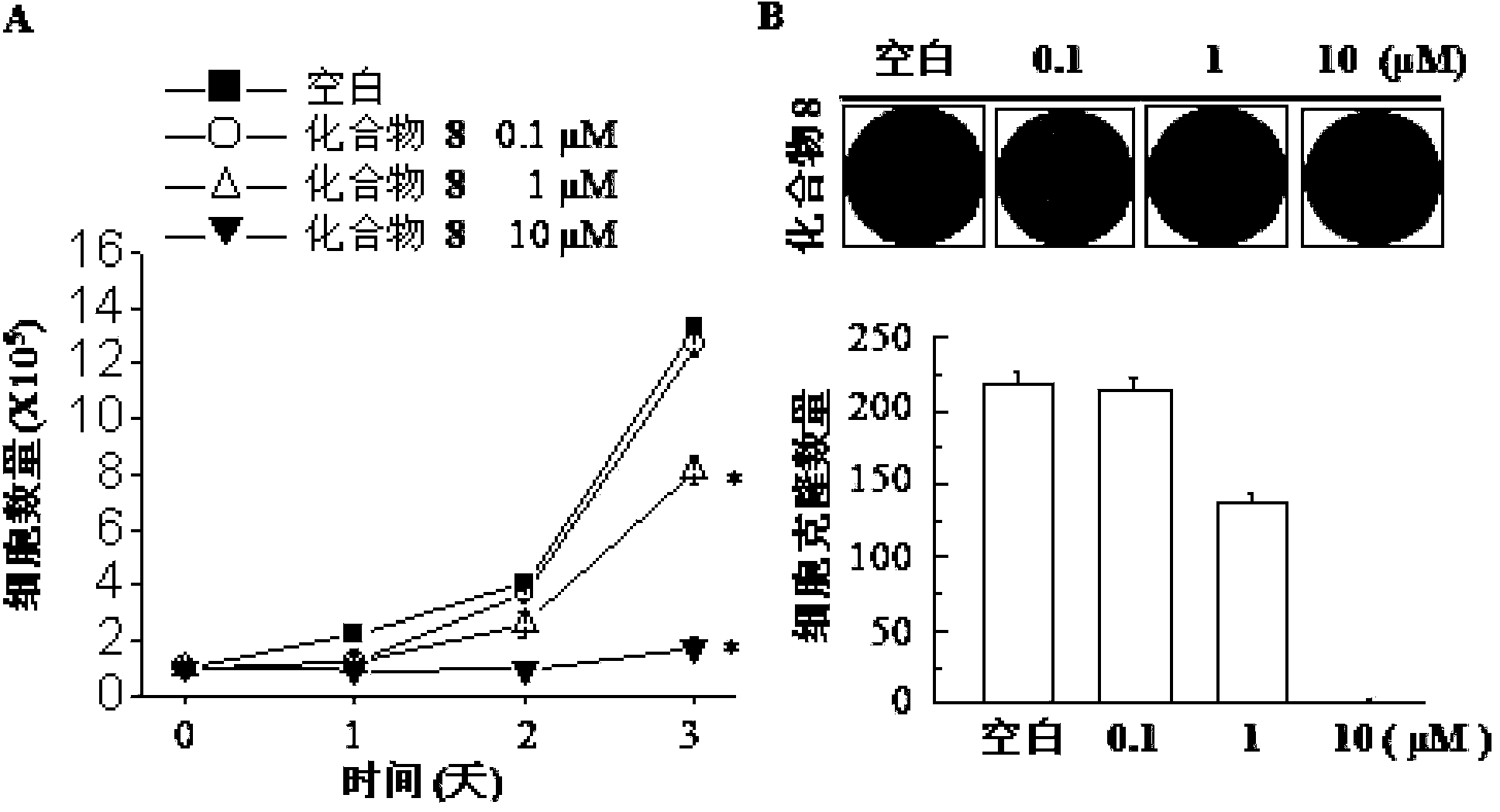
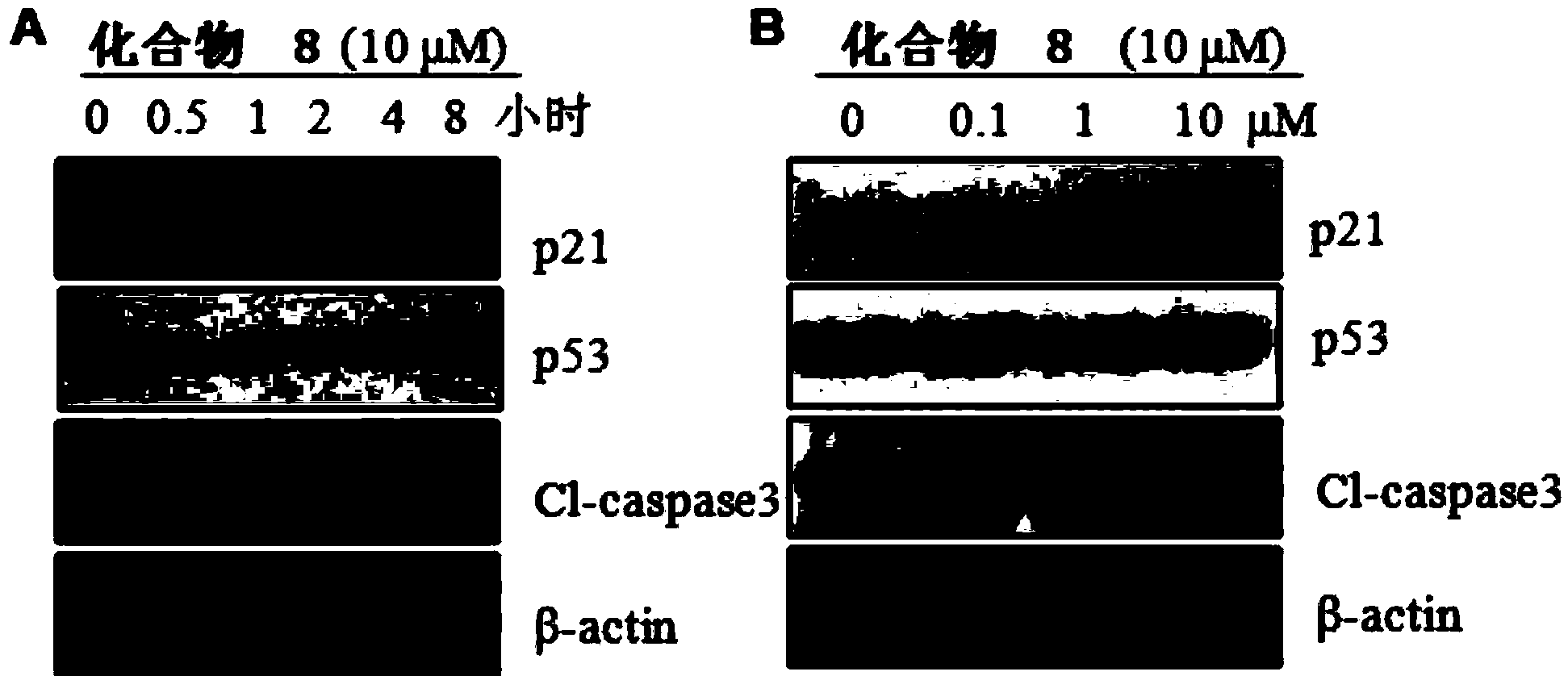
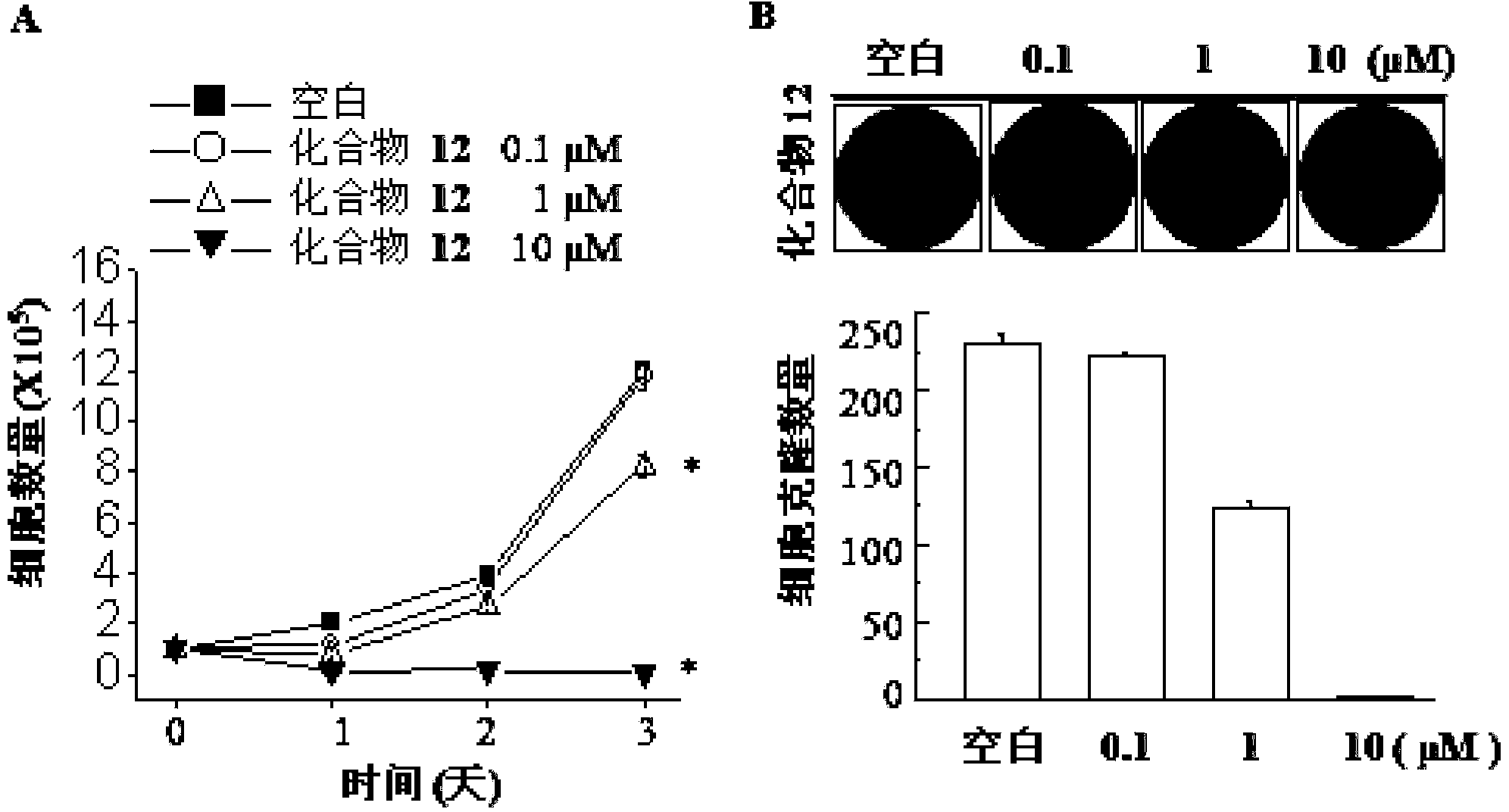
Image
Examples
preparation example Construction
[0041] The preparation method of substituted diaryl ether compound of the present invention, its reaction process is as follows:
[0042]
[0043] That is, under the action of a base, the substituted halogenated benzene or substituted aniline shown in the compound (II) is reacted with the substituted ethyl benzoate shown in the compound (III) to generate the compound (IV), and the compound (IV) is hydrolyzed or Condensation reaction with substituted amine after acid chlorination to obtain compound (I), namely the substituted diaryl ether compound of the present invention.
[0044] Concretely include the following steps:
[0045] (1) The synthetic method of intermediate acid:
[0046]
[0047] (2) The synthesis method of the target compound, including two kinds of A and B, wherein A is as follows:
[0048]
[0049] B is as follows:
[0050]
Embodiment 1
[0052] Example 1: Synthesis of 4-(4-fluorophenoxy)benzoic acid (B1)
[0053]
[0054] 1.0mL (8.9moL) of 1-fluoro-4-iodobenzene, 1.85g (13.4moL) of p-hydroxybenzoic acid, 170mg (0.9mmoL) of cuprous iodide, and 276mg (2.7mmoL) of N,N-dimethylglycine Dissolve 5.81g (17.8moL) of cesium carbonate in 25mL of 1,4-dioxane, heat and stir at 90°C for 24 hours under the protection of argon. After the reaction was completed, the solvent was evaporated under reduced pressure, water was added, and ethyl acetate was extracted three times. The organic layer was washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was removed and separated by silica gel column chromatography (ethyl acetate:petroleum ether=1:30) to obtain a yellow liquid. 1.0 g (4.1 moL) of the synthesized ethyl 1-fluoro-4-phenoxybenzoate was dissolved in 8 mL of ethanol and 8 mL of water, 1.82 g (4.5 moL) of sodium hydroxide was added, and heated to reflux for 3 hours. After completion of the r...
Embodiment 2
[0057] Embodiment 2: the synthesis (B2) of 4-phenoxybenzoic acid
[0058]
[0059] The synthesis method of Example 1 above gave B2 white solid, and the two-step yield was 87%.
[0060] 1 H NMR (600MHz, DMSO-d 6 )δ: 7.02(d, J=8.8Hz, 2H), 7.11(d, J=7.7Hz, 2H), 7.23(t, J=7.3Hz, 1H), 7.45(t, J=7.5Hz, 2H) , 7.95 (d, J=8.8Hz, 2H), 12.82 (br s, 1H) ppm;
[0061] 13 C NMR (150MHz, DMSO-d 6 )δ: 117.2, 119.9, 124.7, 125.2, 130.3, 131.7, 155.1, 161.0, 166.8ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com