Composition with function of removing chloasma and preparation method of composition with function of removing chloasma
A technology for removing chloasma and a composition, which is applied in the directions of medical preparations containing active ingredients, food preparation, and drug combinations, etc., can solve the problem of unclear reasons for melanocyte function, affecting the work and life of patients, and complex pathogenesis. and other problems, to achieve the effect of simple and feasible preparation process, improvement of facial chloasma, and strong pertinence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
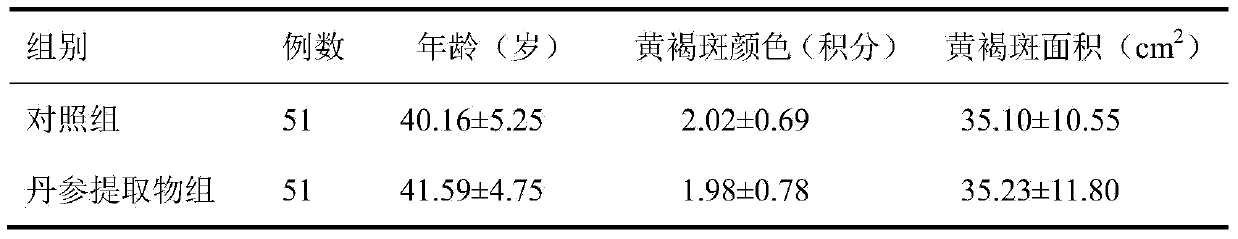
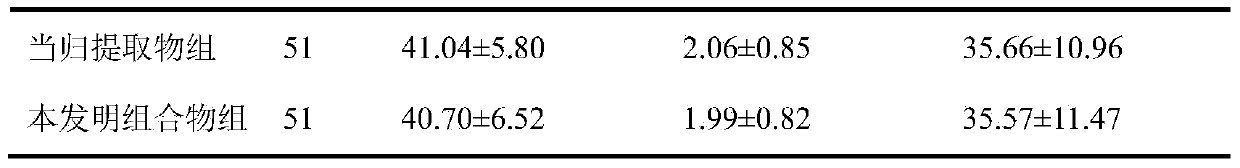
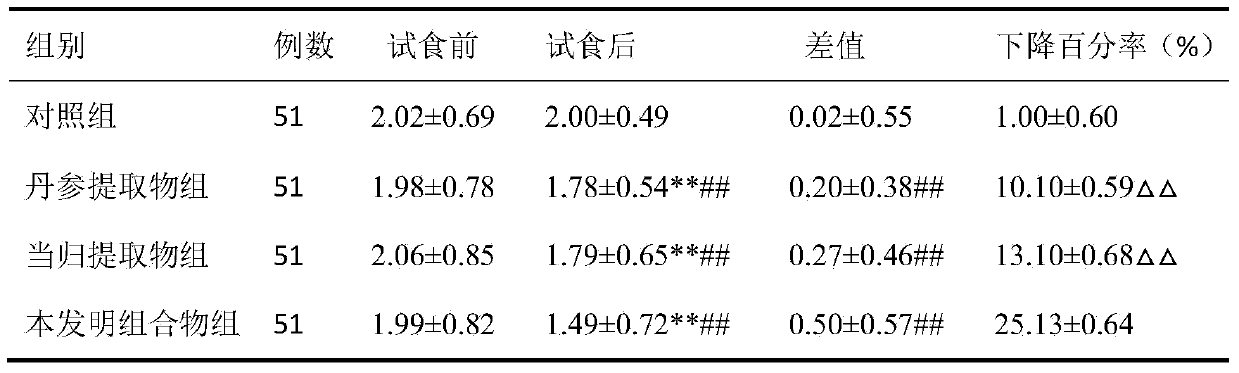
[0034] Formula: Salvia miltiorrhiza extract 117g, angelica extract 67g, rose flower extract 50g, lycopene 17g, vitamin E powder 17g, microcrystalline cellulose 274g, crospovidone 25g, croscarmellose sodium ( (additional) 30g, magnesium stearate (additional) 3g, film coating premix 18g.
[0035] Preparation:
[0036] 1. The lycopene in the formula is passed through a 40-mesh sieve, and the rest of the raw and auxiliary materials are respectively passed through a 80-mesh sieve for later use. The production water is purified water, which should meet the requirements of purified water in the "Chinese Pharmacopoeia" 2010 edition, and the ethanol used in production is edible grade ethanol, which should meet the requirements of GB 10343-2008 edible alcohol.
[0037] 2. Accurately weigh the salvia miltiorrhiza extract, angelica extract, rose flower extract, lycopene, vitamin E powder, microcrystalline cellulose and cross-linked povidone according to the dosage of the formula, mix the...
Embodiment 2
[0043] Formula: Salvia miltiorrhiza extract 175.5g, angelica extract 33.5g, rose flower extract 42g, lycopene 8.5g, vitamin E powder 8.5g, dextrin 232g.
[0044] Preparation method: mix the raw and auxiliary materials evenly, sieve, granulate to make granules, pack and inspect to obtain products that meet the standards.
Embodiment 3
[0046] Formula: Salvia miltiorrhiza extract 58.5g, angelica extract 83.5g, rose flower extract 75g, lycopene 25.5g, vitamin E powder 25.5g, microcrystalline cellulose 89g, magnesium stearate 3g.
[0047] Preparation method: mix the raw and auxiliary materials evenly, pour into capsules to make capsules, pack and inspect to obtain products that meet the standards.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com