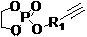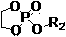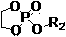Polyphosphoester-based folate-targeted acid-sensitive core-crosslinked drug-loaded micelle and preparation method thereof
A targeted technology of polyphosphate and folic acid, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to prolong the circulation time in the body, good biocompatibility and biodegradability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Polyphosphate block copolymer (PBYP- b -PEOP-OH) Preparation
[0063] Dry the ampoule with a stirrer in an oven at 120°C for at least 24 hours, take it out, connect the ampoule to the double-row tube, pump it with an oil pump, and cool it to room temperature, repeat the pumping and inflation three times, and finally fill it with nitrogen. Next, add 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 0.2738 g, 0.18 mmol), dichloromethane (CH 2 Cl 2, 1.5 mL), isopropanol (IPA, 0.1121 g, 0.12 mmol) and the monomer 2-ynbutyl-2-oxo-1, 3, 2-dioxaphospholane (BYP, 0.8453 g, 4.8 mmol), put the reaction vial into an oil bath set at 25°C, and react under stirring for 30 minutes. After the reaction, the monomer 2-ethyl-2-oxo-1, 3, 2-dioxaphospholane (EOP, 0.73 g, 4.8 mmol) was added, and the reaction was continued in an oil bath at 25°C for 30 minute.
[0064] After the ring-opening reaction, the product was concentrated, and then the concentrated solution was dropped into me...
Embodiment 2
[0065] Embodiment two: the polyphosphate block copolymer (PBYP- b -PEOP-FA) Preparation
[0066] After treating the branched vial and the ground glass stopper equipped with a stirring bar according to the method of Example 1, it was filled with nitrogen, and under the condition of nitrogen, folic acid (FA, 0.0159 g, 0.036 mmol), dimethyl sulfoxide (DMSO, 10 mL), dicyclohexylcarbodiimide (DCC, 0.0096 g, 0.0468 mmol), N -Hydroxysuccinimide (NHS, 0.0054 g, 0.0468 mmol) and 4-dimethylaminopyridine (DMAP, 0.0057 g, 0.0468 mmol). The reaction bottle was filled with nitrogen, and the reaction was stirred at 25°C for 12 hours. After the reaction, weigh the PBYP 43 - b -PEOP 41 -OH (0.4161 g, 0.0300 mmol) was added to the reaction liquid, and the reaction was continued for 24 hours.
[0067] After the reaction, the product was transferred to a molecular weight cut-off of 3500 g·mol -1 In a dialysis bag, dialyze in deionized water for 24 hours, freeze-dry to obtain a light yellow...
Embodiment 3
[0068] Example Three: Acid-sensitive Tetraethylene Glycol (N 3 - a -TEG- a -N 3 ) preparation
[0069] Weigh tetraethylene glycol (TEG, 0.97 g, 5 mmol) and pyridinium 4-methylbenzenesulfonate (PPTS, 0.2513 g, 1 mmol) into a 100 mL vial equipped with a magnetic stirring bar, add toluene ( 20 mL), azeotropically remove water twice, and then dissolve the azeotropic mixture in anhydrous dichloromethane (30 mL), slowly add 2-chloroethyl vinyl ether (CEVE, 2.5 mL , 25 mmol) and anhydrous dichloromethane (10 mL) into a branched vial, after the dropwise addition, the reaction was stirred in an ice-water bath for 0.5 hours.
[0070] After the reaction was completed, 5% sodium carbonate aqueous solution was added to terminate the reaction, diluted with dichloromethane (30 mL), and then saturated sodium chloride phosphate buffer solution (pH 10.0, 10 mL) was added. The lower organic phase was removed, and the aqueous phase was extracted with dichloromethane (20 mL). The organic phas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com