Preparation method of bromfenac sodium
A technology of bromfenac sodium and phosphoric acid, which is applied in the field of medicinal chemistry, can solve the problems that the content of bromfenac sodium cannot be effectively controlled, the appearance and properties of the product are difficult to meet the standards, and the impurity content of the hydrolyzate is high, so as to achieve good appearance and properties, reduce the production, The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The investigation of reaction solvent in the preparation intermediate Ⅳ:
[0057] The present inventors used dichloromethane in the prior art as the reaction solvent to prepare intermediate IV, and found that the post-treatment process was cumbersome, so other reaction solvents were investigated, and the results are shown in Table 1. It can be seen from Table 1 that when dichloromethane is used as the reaction solvent, the reaction time is as long as 24h, and the unrefined purity of intermediate IV obtained is only 87.83%; while N,N-dimethylformamide (DMF) is used as the solvent , the reaction time can be shortened to 3.0h, and the unrefined purity can reach 93.61%, which is slightly higher than the purity of ethyl acetate after refining when using dichloromethane as a solvent; using dimethyl sulfoxide (DMSO) as a solvent, the reaction time can be Shortened to 5.0h, the unrefined purity can reach 92.37%, which is equivalent to the purity obtained when dichloromethane is...
Embodiment 2
[0062] The effect of the addition method of phosphoric acid on the preparation of intermediate III:
[0063] The present inventor investigates the way of adding phosphoric acid, and the results are shown in Table 2. As can be seen from Table 2, using dropwise addition of phosphoric acid, adding phosphoric acid at one time, adding phosphoric acid in two batches or adding phosphoric acid in three batches, the reaction time is longer, and the crystallization product is viscous and difficult to filter; while adding phosphoric acid in four batches When phosphoric acid is used, the reaction time is shortened to 11-12 hours, and the crystallization product is easy to filter; when phosphoric acid is added in five batches, although the crystallization product is easy to filter, the reaction time needs to be 17-18 hours.
[0064] Table 2: The effect of the addition method of phosphoric acid on the preparation of intermediate Ⅲ
[0065]
Embodiment 3
[0067] Effects of different recrystallization solvents on the product quality of intermediate Ⅲ and bromfenac sodium:
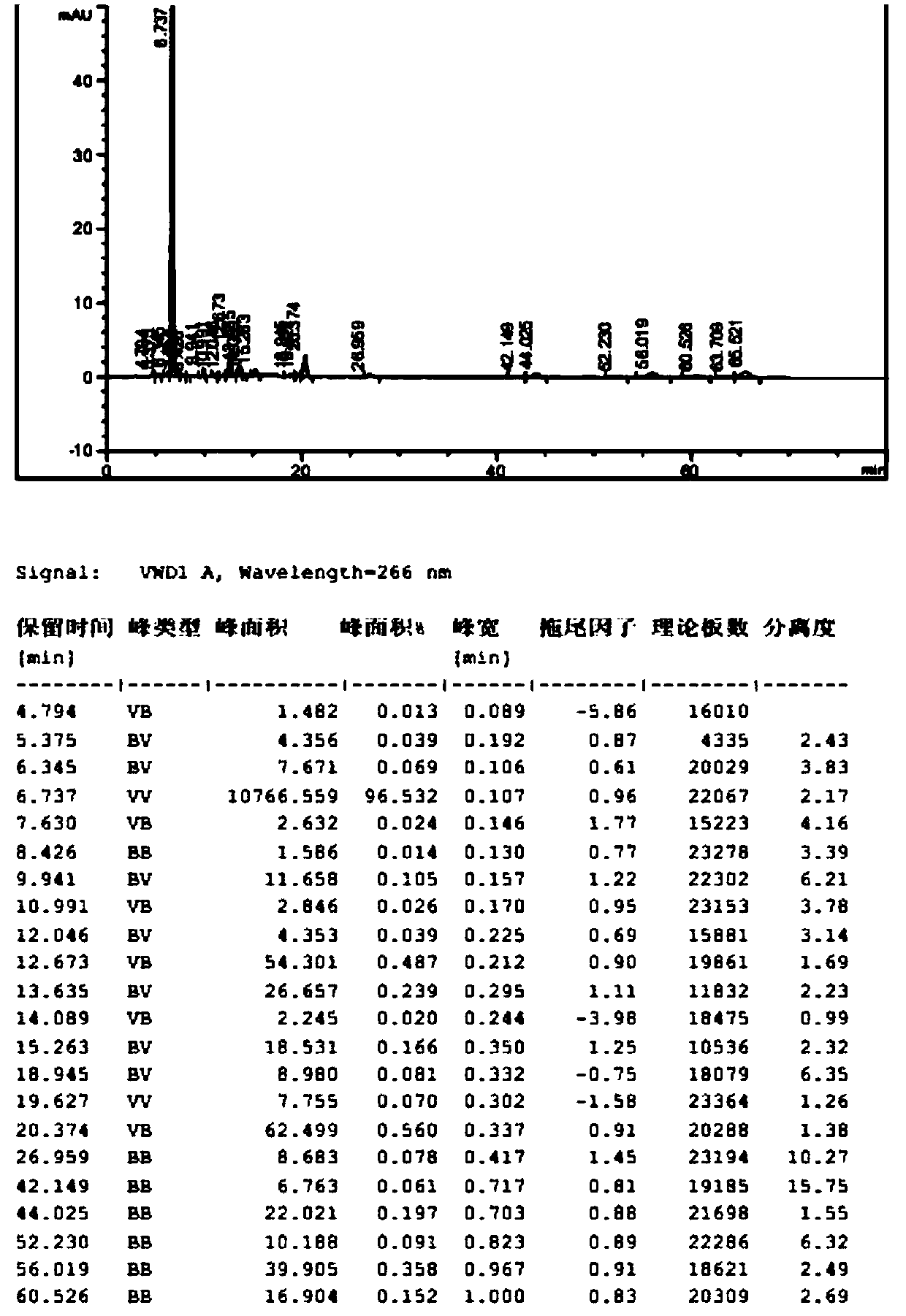
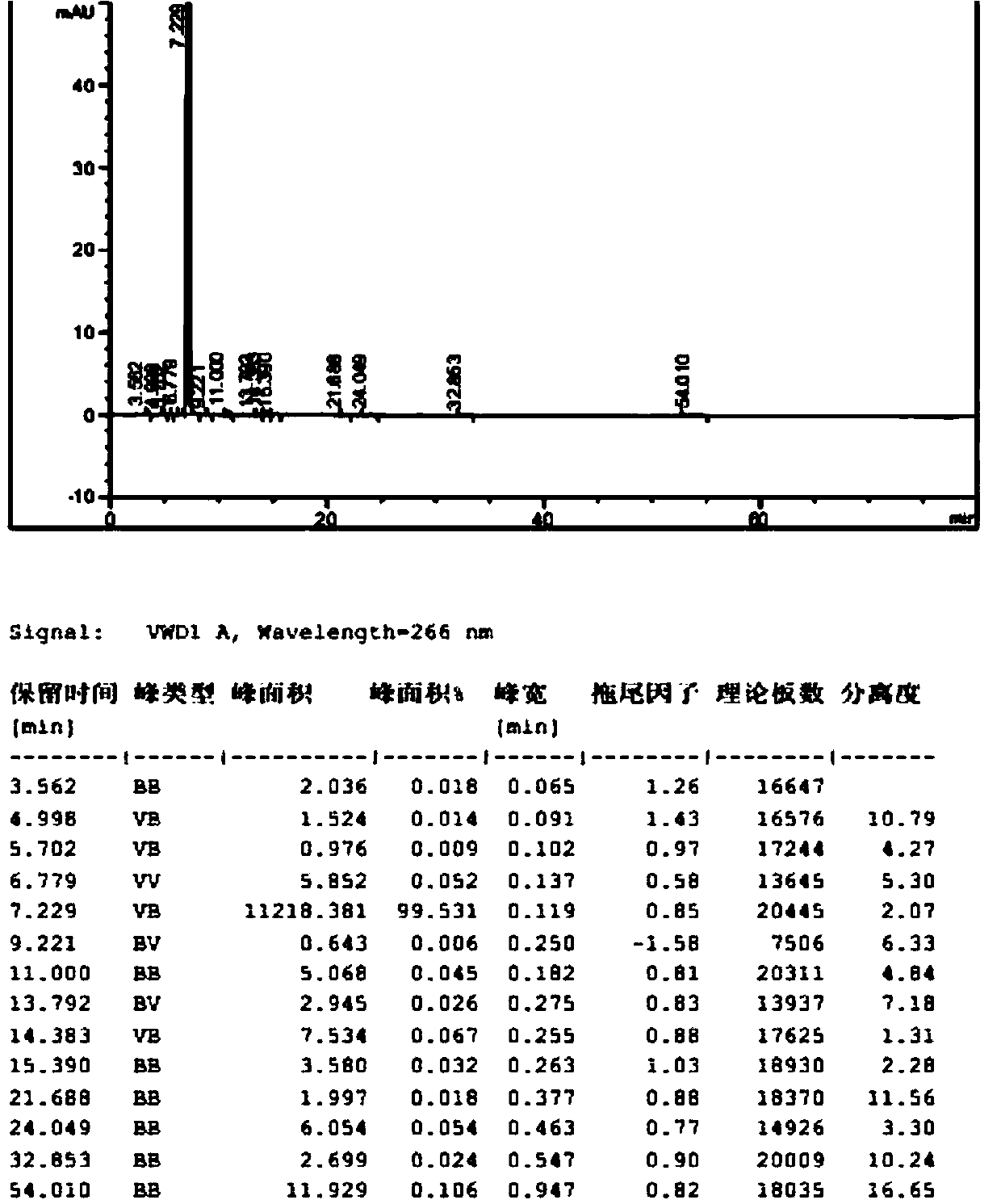
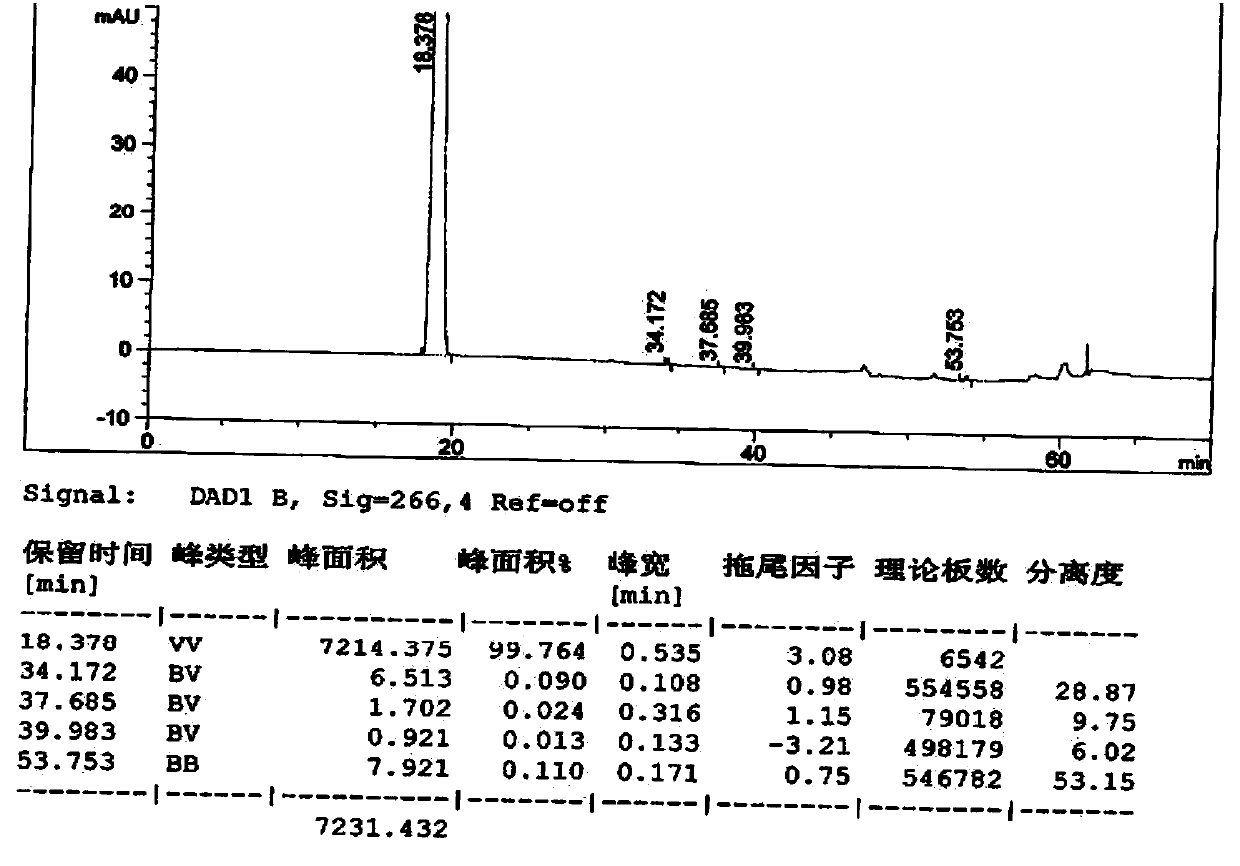
[0068] The inventor found through a large number of experiments that the content of unknown impurities with a relative retention time of about 2.1 in the bromfenac sodium prepared by the prior art is relatively high, which is caused by the unknown impurities with a relative retention time of about 1.95 in intermediate III. If intermediate III with relative retention time of about 1.95 and unknown impurity content above 0.2% is used for follow-up reaction, even if intermediate II and bromfenac sodium are refined using prior art, it cannot be used The content of unknown impurities with a relative retention time of about 2.1 in bromfenac sodium was reduced to below 0.1%. The inventors investigated various recrystallization solvents, and the results are shown in Table 3. As can be seen from Table 3, the impurity removal effect of single organic solvents such as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com