Red phosphorescence iridium complexes, preparing method thereof and organic electroluminescence device
A technology of iridium metal complexes and red phosphorescence, applied in the fields of electric solid-state devices, organic chemistry, luminescent materials, etc., can solve the problems of low efficiency and difficulty in obtaining satisfactory luminescent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] see figure 1 , the preparation method of the red phosphorescence iridium metal complex of an embodiment, comprises the steps:
[0053] Step S110: In the first inert gas atmosphere, Ar-Br and acenaphthyl-5-boronic acid are dissolved in the first solvent at a molar ratio of 4:4.5-5, and a catalyst and a carbonate solution are added to carry out a Suzuki coupling reaction After 8-12 hours, after separation and purification, the ring metal main ligand acenaphthyl-5-ylbenzodiazepine six-membered ring is obtained.
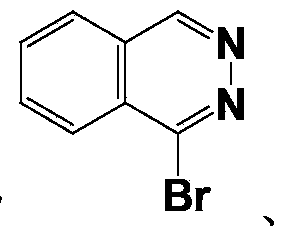
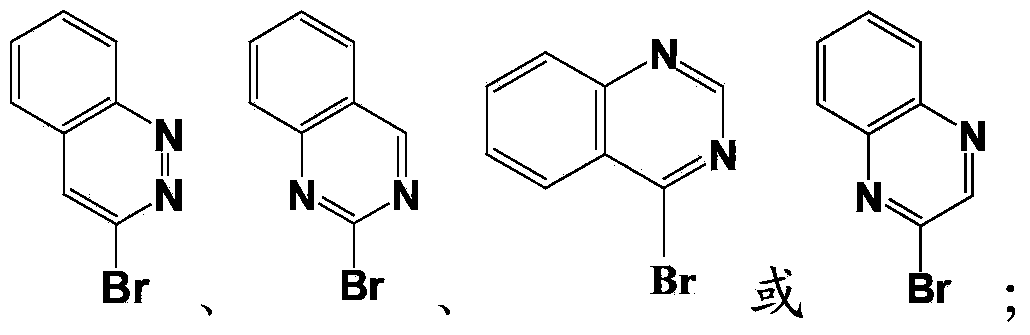
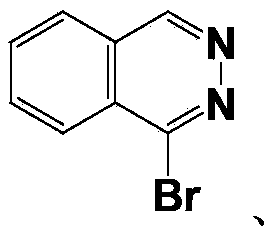
[0054] Wherein, Ar is phthalazine, cinnoline, benzopyrimidine or quinoxaline.
[0055] The first inert gas is argon, helium or neon.
[0056] The first solvent is toluene or tetrahydrofuran. The amount of the first solvent is suitable to fully dissolve Ar-Br and acenaphthylene-5-boronic acid. The catalyzer is a palladium catalyst, preferably tetrakistriphenylphosphine palladium (Pd(pph 3 ) 4 ) or palladium dichlorobistriphenylphosphine (PdCl 2 (PPh 3 ) 2 ...
Embodiment 1
[0088] Red Phosphorescent Bis[1-(Acenaphthyl-5-yl)phthalazine-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione) iridium complex synthesis.
[0089] Red Phosphorescent Bis[1-(Acenaphthyl-5-yl)phthalazine-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione) iridium complex has the following structural formula:
[0090]
[0091] (1) Synthesis of 1-(acenaphthyl-5-yl)phthalazine
[0092]In an argon atmosphere, 0.84g (4mmol) of 1-bromophthalazine, 0.95g (4.8mmol) of acenaphthene-5-boronic acid and 0.23g (0.20mmol) of tetrakistriphenylphosphine palladium were dissolved in 30mL of toluene, followed by 10 mL of an aqueous solution containing 0.85 g (8 mmol) of sodium carbonate was added dropwise to the reaction system. Heated and stirred under reflux for Suzuki coupling reaction for 10h. After the reaction is complete, the reaction solution is naturally cooled to room temperature, extracted several times with an appropriate amount of water and ethyl acetate, the organic phase is dried wi...
Embodiment 2
[0121] Red phosphorescent bis[3-(acenaphthyl-5-yl)cinnoline-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione) iridium complex synthesis.
[0122] Red phosphorescent bis[3-(acenaphthyl-5-yl)cinnoline-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione) iridium complex has the following structural formula:
[0123]
[0124] (1) Synthesis of 3-(acenaphthyl-5-yl)cinnoline
[0125] In a helium atmosphere, 0.84g (4mmol) of 3-bromocinnoline, 0.99g (5mmol) of acenaphthylene-5-boronic acid and 0.28g (0.24mmol) of tetraphenylphosphine palladium were dissolved in 25mL of tetrahydrofuran, followed by the reaction 10 mL of an aqueous solution containing 1.38 g (10 mmol) of potassium carbonate was added dropwise to the system. Heated and stirred under reflux for Suzuki coupling reaction for 12h. After the reaction was complete, the reaction solution was naturally cooled to room temperature, and extracted several times with appropriate amount of water and ethyl acetate. The organic phase was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com