Stable flurbiprofen axetil micro-nano-emulsion and preparation method thereof
A technology of flurbiprofen axetil and micro-nano emulsion, which is applied in the fields of emulsion delivery, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc. Pain and other problems, to achieve good thermodynamic stability, small and uniform particles, reduce the effect of injection pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
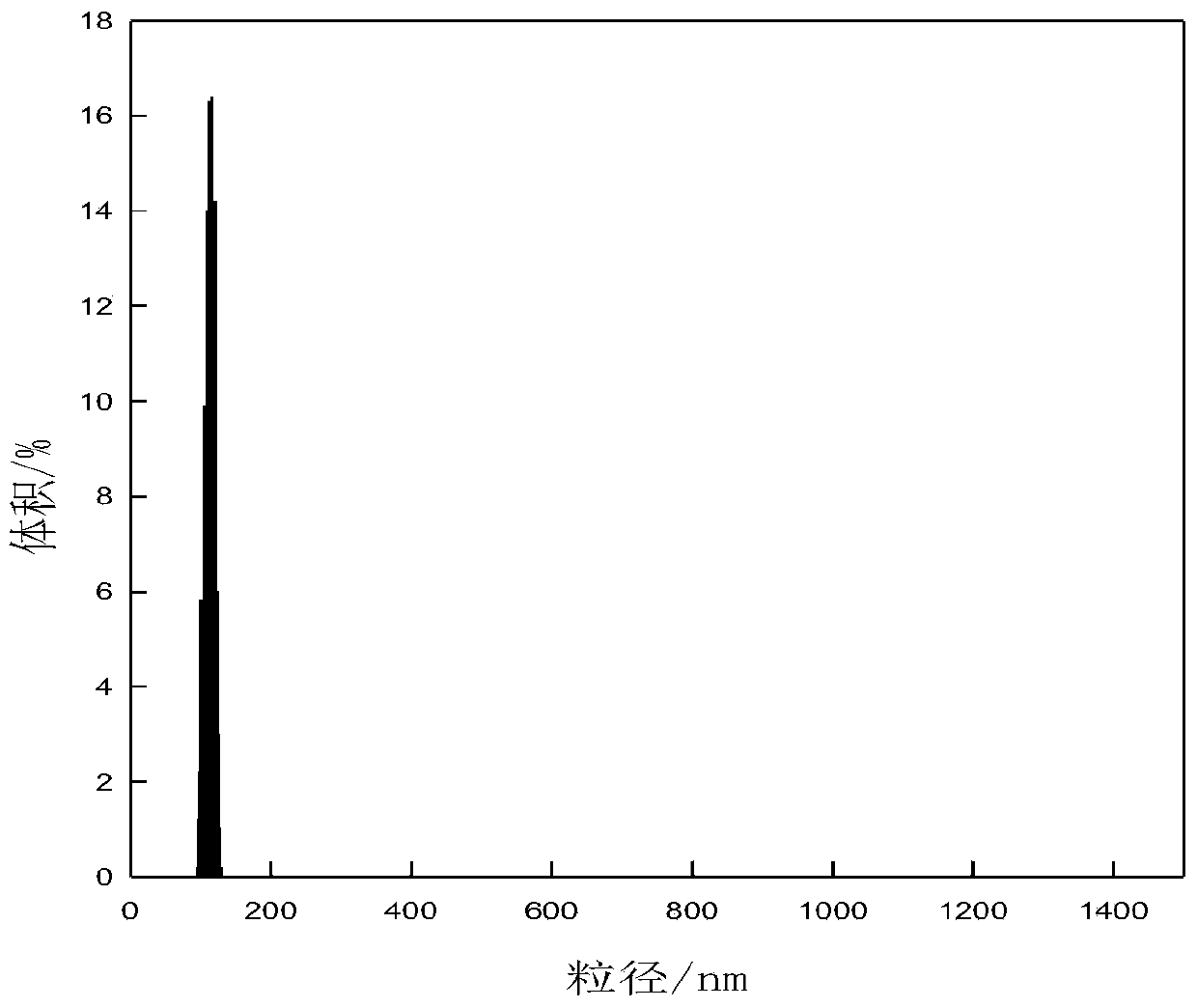
Image
Examples
Embodiment 1
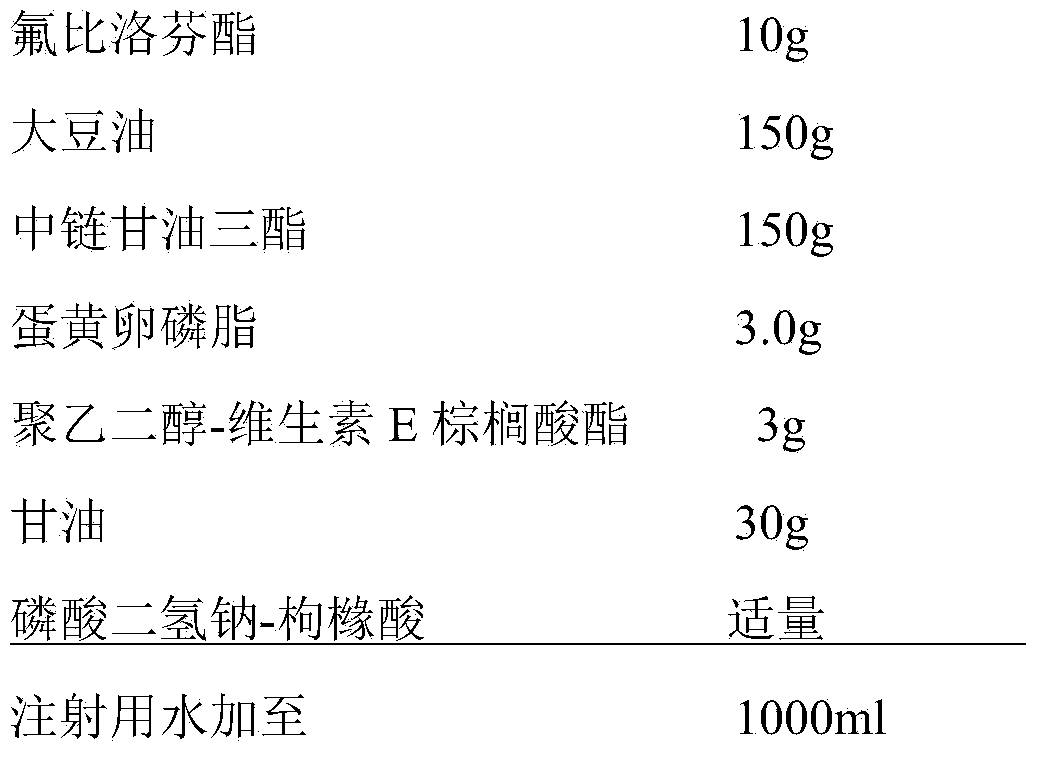
[0039] prescription
[0040]
[0041] Process:
[0042] (1) Preparation of the oil phase: Take 3.0g egg yolk lecithin, 3g polyethylene glycol-tocopherol palmitate, add 150g soybean oil and 150g medium chain triglycerides, and stir at 30°C under nitrogen protection 20min to fully dissolve, then add 10g flurbiprofen axetil, dissolve and mix evenly, as the oil phase;
[0043] (2) Preparation of the water phase: under the conditions of nitrogen protection and a temperature of 30°C, take 500 ml of water for injection, add 30 g of glycerin, and stir to dissolve it as the water phase;
[0044] (3) Preparation of colostrum: under nitrogen protection and temperature 30℃, add step (1) oil phase to step (2) water phase, high-speed shear dispersion, shear speed 10000rpm, time 15 minutes to form Colostrum, and adjust the total amount to 1000ml with water for injection;
[0045] (4) High-pressure homogenization: Under nitrogen protection and a temperature of 30°C, the colostrum obtained in step (3)...
Embodiment 2
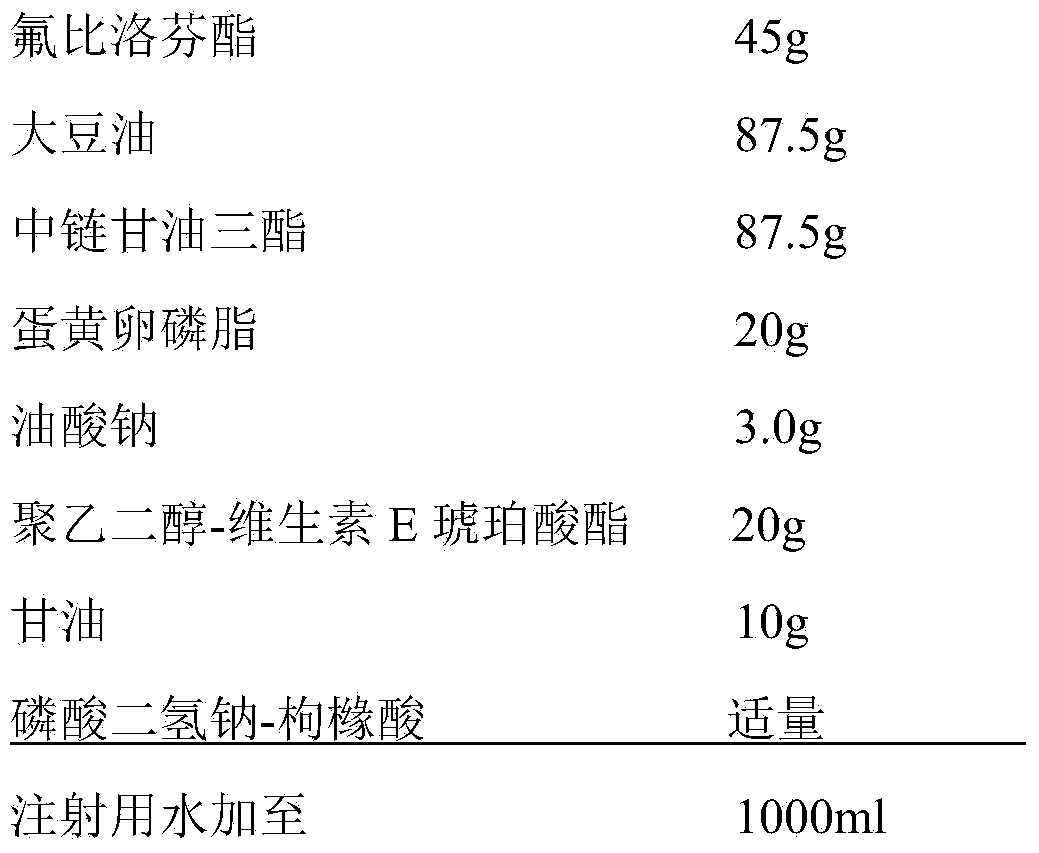
[0049] prescription
[0050]
[0051] Process:
[0052] (1) Preparation of the oil phase: take 20g egg yolk lecithin, 3.0g sodium oleate, 20g polyethylene glycol-tocopherol succinate and add 87.5g soybean oil and 87.5g medium chain triglycerides, in a nitrogen protected Under the conditions, stir at 40°C for 20 minutes to fully dissolve, then add 45g flurbiprofen axetil, dissolve and mix well, as the oil phase;
[0053] (2) Preparation of water phase: Take 500ml of water for injection under the condition of nitrogen protection and temperature of 40°C, add 10g of glycerin, stir to dissolve it, as the water phase;
[0054] (3) Preparation of colostrum: under nitrogen protection and temperature 40℃, add step (1) oil phase to step (2) water phase, high-speed shear dispersion, shear speed 8000rpm, time 35 minutes to form Colostrum, and adjust the total amount to 1000ml with water for injection;
[0055] (4) High-pressure homogenization: Under nitrogen protection and a temperature of 40°C, ...
Embodiment 3
[0058] prescription
[0059]
[0060] Process:
[0061] (1) Preparation of the oil phase: take 11g egg yolk lecithin, 6.0g sodium oleate, 40g polyethylene glycol-tocopherol palmitate and add 25g soybean oil and 25g medium chain triglycerides, under nitrogen protection , Stir at 20°C for 20min to fully dissolve, then add 80g flurbiprofen axetil, dissolve and mix well, as the oil phase;
[0062] (2) Preparation of the water phase: under the condition of nitrogen protection and temperature of 20°C, take 500ml of water for injection, add 50g of glycerin, stir to dissolve it, and serve as the water phase;
[0063] (3) Preparation of colostrum: under the condition of nitrogen protection and temperature of 20℃, add the oil phase of step (1) to the water phase of step (2), high-speed shear dispersion, shear speed 7000rpm, time 40 minutes, and form Colostrum, and adjust the total amount to 1000ml with water for injection;
[0064] (4) High-pressure homogenization: Under the conditions of nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com