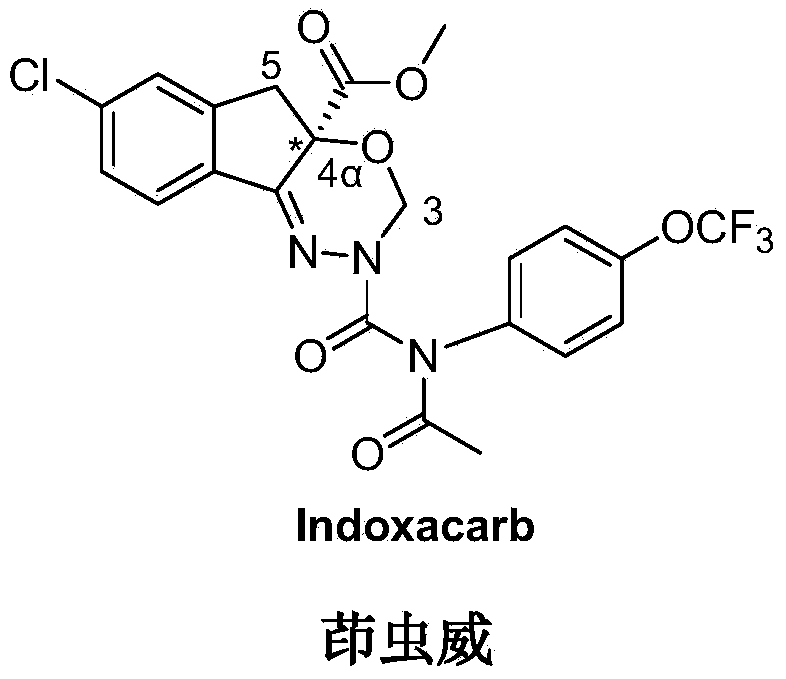
Preparation method for novel insecticide indoxacarb
A technology of indoxacarb and insecticide, which is applied in the field of preparation of new insecticide indoxacarb, can solve problems such as danger, and achieve the effects of good purity, good product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
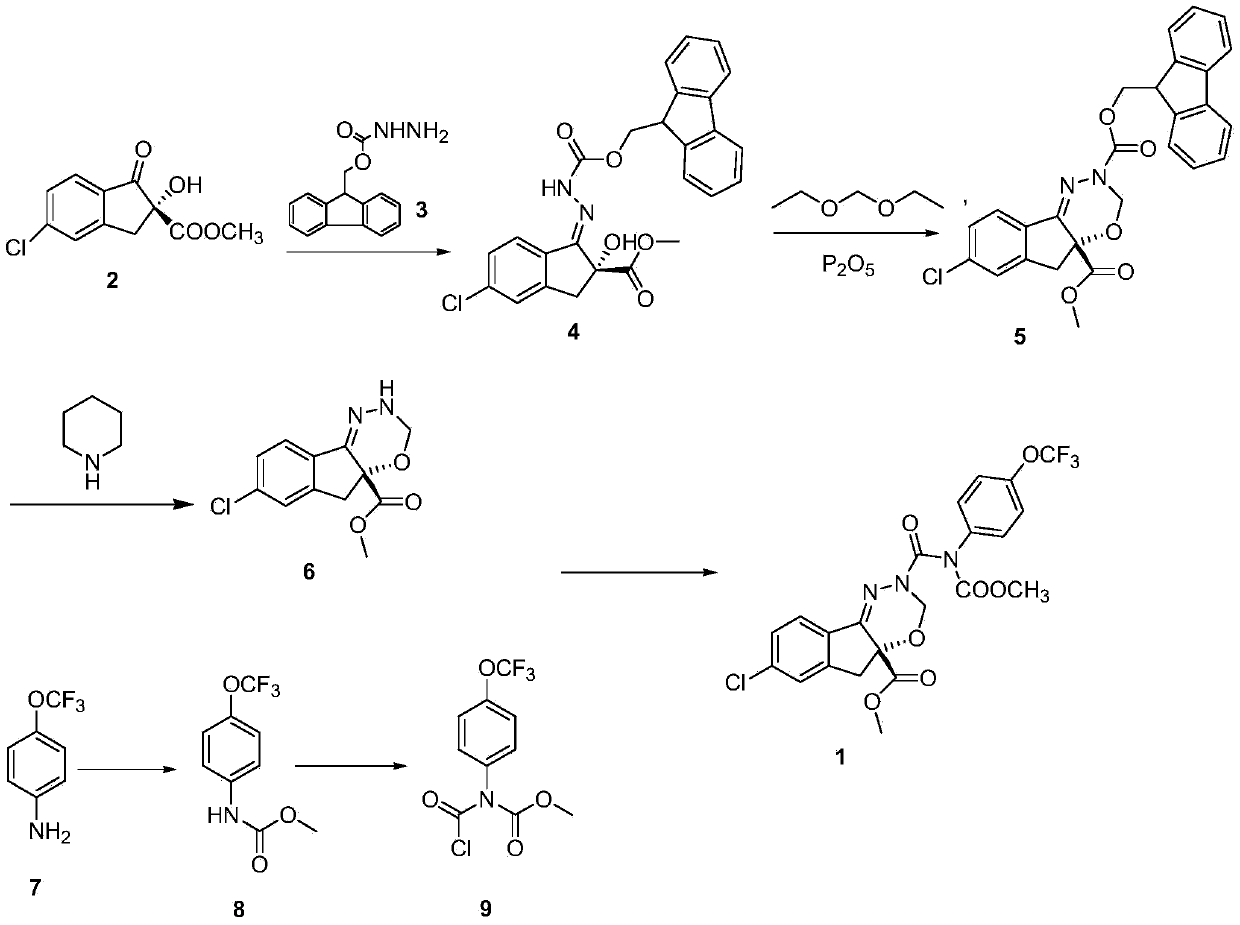
Examples
example 1
[0021] Example 1: Preparation of hydrazine carboxylate fluorene methyl ester
[0022] 80% hydrazine hydrate (24.5g, 0.40mol) was added to a 500mL reaction flask, and a solution of Fmoc-Cl (51.8g, 0.20mol) in tetrahydrofuran (280ml) was added dropwise at -20°C. After the dropwise addition was completed in about 2 hours , then continue to stir for 30min, stop the reaction, pour it into a separatory funnel, stand still, separate the upper organic layer, evaporate the solvent under reduced pressure, cool the residue, and filter to obtain the crude hydrazine carboxylic acid fluorene methyl ester, which is washed with ethyl acetate. After recrystallization, 32.7 g of hydrazine carboxylate fluorene methyl ester was obtained as white needle crystals. Yield: 85.7%, melting point: 168-170°C. 1 HNMR(DMSO)δ(ppm): 4.05(s, 2H), 4.16-4.31(m, 3H), 7.31(t, 2H, J=7.3Hz), 7.40(t, 2H, J=7.3Hz), 7.67 (d, 2H, J=7.3 Hz), 7.86 (d, 2H, J=7.3 Hz), 8.36 (s, 1H).
example 2
[0023] Example 2: Preparation of hydrazine carboxylate fluorene methyl ester
[0024]80% hydrazine hydrate (26.8g, 0.60mol) was added to a 500mL reaction flask, and a solution of Fmoc-Cl (51.8g, 0.20mol) in tetrahydrofuran (360ml) was added dropwise at -10°C, and the dropwise addition was completed within about 2 hours. , and then continue to stir for 60 min, stop the reaction, pour it into a separatory funnel, stand still, separate the upper organic layer, remove the solvent by distillation under reduced pressure, cool the residue, and filter to obtain the crude hydrazine carboxylate fluorene methyl ester, which is washed with ethyl acetate. After recrystallization, 33.2 g of hydrazine carboxylate fluorene methyl ester was obtained as white needle crystals. Yield: 87.0%.
example 3
[0025] Example 3: Preparation of (+)[5-chloro-2,3--hydrogen-2-hydroxy-2-methoxycarboxy-1H-indene-ylidene]hydrazine carboxylate fluorene methyl ester
[0026] Into a 500mL reaction flask, add (+) methyl 5-chloro-1,3-dihydro-2-hydroxy-1-oxo-2H-indene-2-carboxylate (24.1g, 0.10mol), hydrazine carboxylic acid Fluorene methyl ester (25.3 g, 0.10 mol), p-toluenesulfonic acid (3.0 g, 0.015 mol) and methanol (250 ml). Heated reflux reaction for 20h, after the reaction,
[0027] Cool and filter to obtain) [5-chloro-2,3--hydrogen-2-hydroxy-2-methoxycarboxy-1H-indene-idene] hydrazine carboxylic acid fluorene methyl ester crude product, recrystallize from methanol, filter , dried in vacuo to give a white solid [5-chloro-2,3--hydrogen-2-hydroxy-2-methoxycarboxy-1H-indene-idene]hydrazine carboxylate fluorene methyl ester 39.5g, yield For 82.8%, melting point: 172-174 ℃. 1 HNMR (CDCl 3 ), δ(ppm): 3.633(d, 1H, J=5.7Hz), 3.812(d, 1H, J=5.7Hz), 4.122(s, 3H), 4.532(m, 1H), 4.754(m, 2H) ), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com