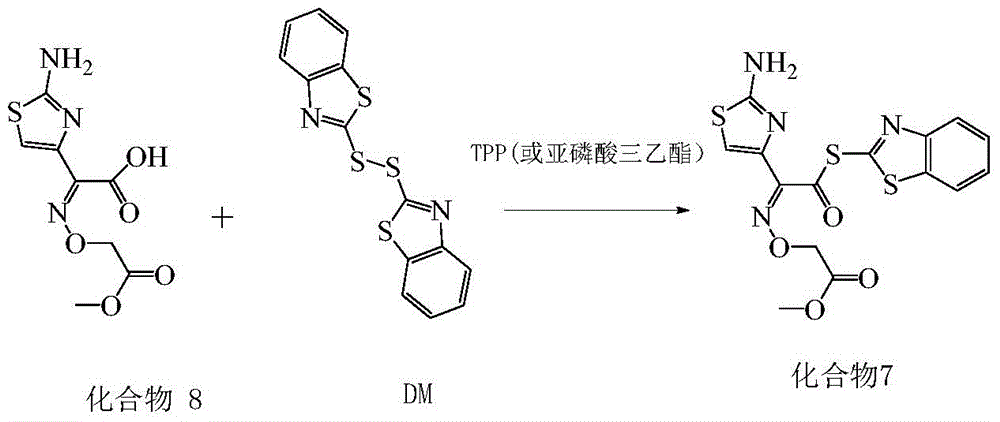
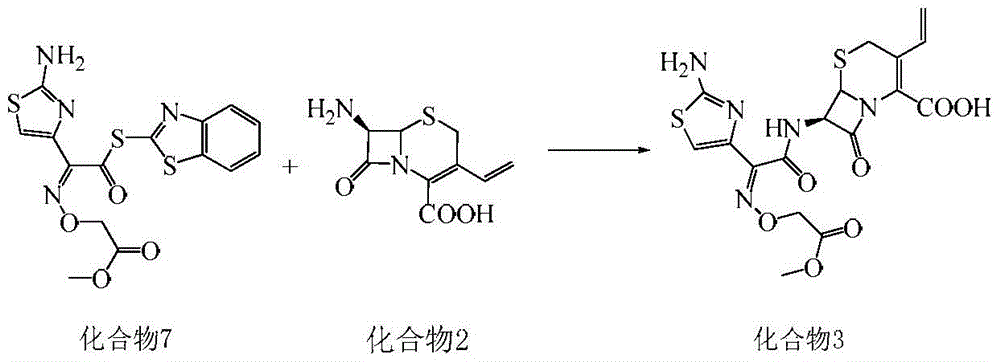
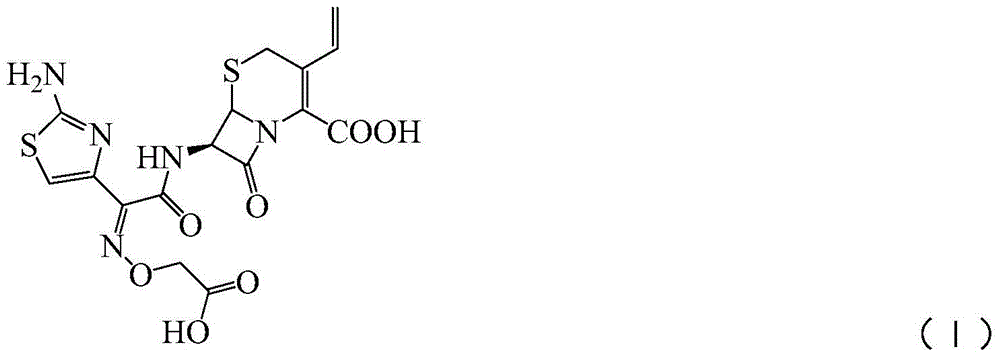A kind of synthetic method of cefixime
A technology of cefixime and synthetic method, which is applied in the field of chemical pharmacy, can solve problems such as difficult removal, and achieve the effects of easy removal, reduced loss, and improved color and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] (1) Add 150g MICA (0.579mol) into a 2000mL four-necked reaction flask, then add 400ml dichloromethane, 0.2g triethylenediethylamine (CAS: 280-57-9) and 107.3g tri-n-butylamine ( 0.579mol), stirring and cooling down to -5°C, and controlling the temperature of the material. Add 132 g (0.7 mol) of diethylphosphoryl thiochloride dropwise. After the addition is complete, keep the temperature at 0° C. to 5° C. and react for 2 to 3 hours. The temperature was controlled at 20° C., P=-0.95 MPa, and the dichloromethane solvent was distilled off under reduced pressure and low temperature to obtain 200 g of cefixime side-chain parathion active ester in a light orange oily form, with a yield of 84%.
[0079](2) Add 900 mL of tetrahydrofuran and stir to dissolve the orange-yellow cefixime side-chain parathion active ester obtained in step (1), add 80 g of 7-AVCA (0.353 mol), and add 0.7 g of sodium bisulfite. Add 100 mL of aqueous sodium hydroxide solution (prepared with 18.4 g of N...
Embodiment 2
[0086] According to (1) step in the above-mentioned embodiment 1, the orange-yellow cefixime side-chain acid phosphine active ester was obtained, and 900mL tetrahydrofuran was added to stir and dissolve, 80g 7-AVCA (0.353) was added, and 100mL aqueous sodium hydroxide solution (NaOH 18.4 g+700mL water), maintain the temperature at 5-10°C, and keep the reaction for 6 hours. After the reaction was completed, 800 mL of ethyl acetate was added, stirred for 30 minutes, allowed to stand for 60 minutes, and separated into layers; the lower aqueous layer was collected. Add 400 mL of ethyl acetate to the aqueous layer and extract once more. Stir for 20 minutes. Let stand for 60 minutes, and separate layers; the lower aqueous phase is collected into a hydrolysis bottle.
[0087] Cool the water phase to 0°C, add the mixed alkali solution (pre-prepared mixed alkali solution: add 34.5g NaOH and 24.5g sodium bicarbonate to 180mL purified water, stir to dissolve and pre-cool to 0°C-5°C, se...
Embodiment 3
[0089] The orange-yellow cefixime side chain acid phosphion active ester that (1) step makes in the above-mentioned example 1, add 900mL tetrahydrofuran and stir to dissolve, add 80g 7-AVCA (0.353), dropwise add 100mL sodium hydroxide aqueous solution (NaOH 18.4g +700mL water to prepare), maintain the temperature at 5-10°C, and keep the reaction for 6 hours. At the end of the reaction, 800 mL of butyl acetate was added, stirred for 30 minutes, allowed to stand for 60 minutes, and separated into layers; the lower aqueous layer was collected. Add 400 mL of butyl acetate to the aqueous layer and extract once more. Stir for 20 minutes. Let stand for 60 minutes, and separate layers; the lower aqueous phase is collected into a hydrolysis bottle.
[0090] (3) Cool the water phase to 0°C, add the mixed alkali solution (pre-prepared mixed alkali solution: add 34.5g NaOH and 24.5g sodium bicarbonate to 180mL purified water, stir to dissolve and pre-cool to 0°C ~ 5°C, set aside ), the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com