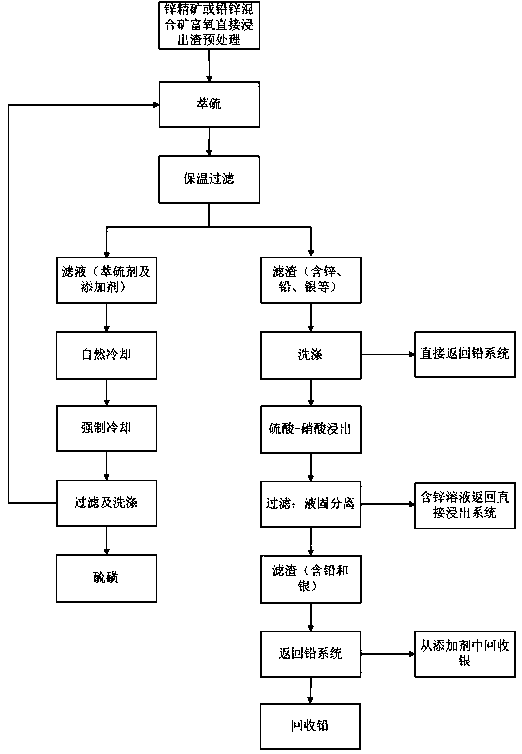
Process for recovering sulfur, lead, zinc and silver from oxygen-enriched directly leached residues of zinc concentrates or lead-zinc mixed ores
A technology for mixing ore and zinc concentrate, applied in the field of silver, zinc, sulfur and lead recovery, can solve the problems of high energy consumption, sulfuric acid pollution, difficult extraction of valuable metals, etc., and achieve high product quality and recovery rate. High, realize the effect of comprehensive utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0025] Composition of sulfur slag (processed through 100 mesh sieve, dry basis): lead 1.22%, zinc 4.75%, indium 0.0029%, silver 0.0569%, sulfur 74.83%.
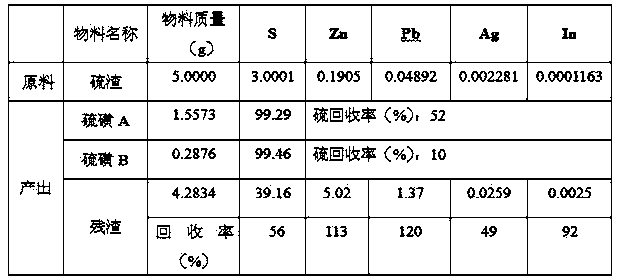
[0026] Weigh 5.0000g of sulfur residue, 1.0000g of A# additive, 62.5ml (100g) of tetrachlorethylene, heat in a water bath at 100°C for 30min, filter immediately to obtain 3.2126g of residue, cool to 27.5°C to obtain 1.8410g of sulfur A, continue to cool to At 9°C, 0.3033 g of sulfur B was obtained. The washed filter residue is directly returned to the lead system to recover lead and silver, and the recovery rates of lead, silver, indium and zinc are 110%, 34%, 89% and 113% respectively.
[0027]
Embodiment approach 2
[0029] Composition of sulfur slag (processed through 100 mesh sieve, dry basis): lead 1.22%, zinc 4.75%, indium 0.0029%, silver 0.0569%, sulfur 74.83%.
[0030] Weigh 10.0000 g of the processed sample sulfur residue, 2.0000 g of A# additive, 125 ml of a mixture of tetrachlorethylene and toluene, heat in a water bath at 100 ° C for 25 min, filter immediately to obtain 6.3962 g of residue, cool to 27.5 ° C, and obtain 3.6703 g of sulfur A, Continue cooling down to 9°C to obtain 0.6098 g of sulfur B. First add the washed filter residue to a jacketed heating device with a mixed solution of dilute sulfuric acid and nitric acid in advance, heat and stir for 1-2 hours, and then return the filter residue to the lead system to recover lead, silver, lead, silver, indium The recoveries of zinc were 114%, 30%, and 79%, respectively; the zinc-containing solution was returned to the direct leaching stage, and the recovery of zinc was 116%.
[0031]
Embodiment approach 3
[0033]Composition of sulfur residue (processed through 120 mesh sieve, dry basis): lead 1.22%, zinc 4.75%, indium 0.0029%, silver 0.0569%, sulfur 74.83%.
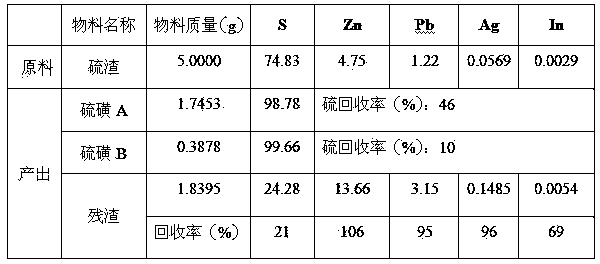
[0034] Weigh 5.0000 g of processed sample sulfur residue, 62.5 ml of trichlorethylene, heat in a water bath at 100°C for 8 minutes, filter immediately to obtain 1.8395 g of residue, cool down to 27.5°C to obtain 1.7453 g of sulfur A, continue cooling to 9°C to obtain sulfur B 0.3878 g. The washed filter residue is directly returned to the lead system to recover lead and silver. The recovery rates of lead, silver, indium and zinc are 95%, 96%, 69% and 106% respectively.
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com