Method for preparing swine epidemic encephalitis B inactivated vaccine
A technology of Japanese encephalitis and inactivated vaccines, applied in the field of vaccines, can solve problems affecting the quality of hamster primary cell batch-to-batch stability, concerns about vaccine side effects and immunity, and incomplete product safety guarantees, and achieve improved Batch-to-batch stability, small destructive effects, and stable immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 The comparison experiment of different cell culture porcine encephalitis virus
[0030] 1. Experimental method:
[0031] Cell preparation: Prepare primary hamster kidney cells according to the "Trial Procedures for the Manufacture and Inspection of Live Attenuated Japanese Encephalitis Vaccine of Porcine Japanese Encephalitis", 96-well culture plate, Chinese hamster lung cells (V79 strain) plated at a concentration of 100,000 to 200,000 / mL .
[0032] Toxic value determination: take the venom for 10-fold serial dilution, take 10 -5 、10 -6 、10 -7 、10 -8 Inoculate plated cells with 4 dilutions of virus liquid, inoculate 5-8 wells for each dilution, and set up non-inoculated control cell wells at the same time, culture at 36-37°C for 7 days, observe cell lesions, and calculate TCID 50 .
[0033] 2. Test results:
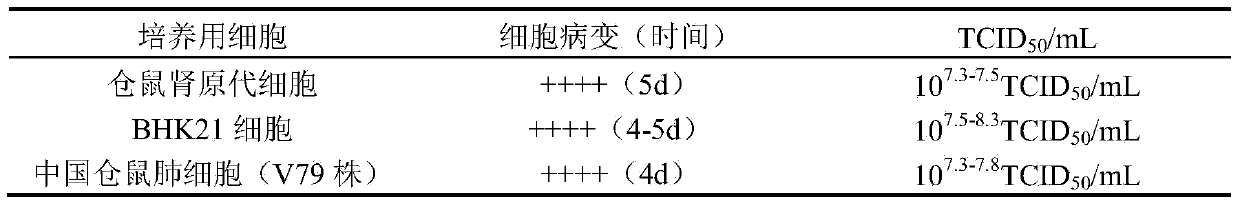
[0034] (1) Comparison of cultured virus titers
[0035] Result analysis: The titer of JEV virus cultured by Chinese hamster lung cells (V79 s...
Embodiment 2
[0040] Example 2 Pathogenicity comparison test of JEV virus after continuous passage on Chinese hamster lung cells
[0041] Take Chinese hamster lung cells (V79 strain) to culture JEV virus, and measure cytopathic TCID respectively 50 and PFU, LD causing mouse death 50 , to compare the virulence changes of the virus after serial passage on the cells.
[0042] The results showed that: JEV virus was continuously cultured in Chinese hamster lung cells for 20 generations, and the virulence changed little, and the virulence did not return to strong (see Table 2).
[0043] Table 2 Changes in virulence of JEV virus after continuous passage on Chinese hamster lung cells (V79 strain)
[0044]
Embodiment 3
[0046] A method for preparing porcine epidemic Japanese encephalitis inactivated vaccine, comprising the following steps:
[0047] (1) Virus seed preparation: select the virus in the hamster lung cell line to adapt to the cell virus seed (JEV P3 strain) identified by cloning, select the first 3 generations of virus as the virus seed for production, and the virus content per milliliter is ≥10 7.5 TCID 50 , Store below -20°C.
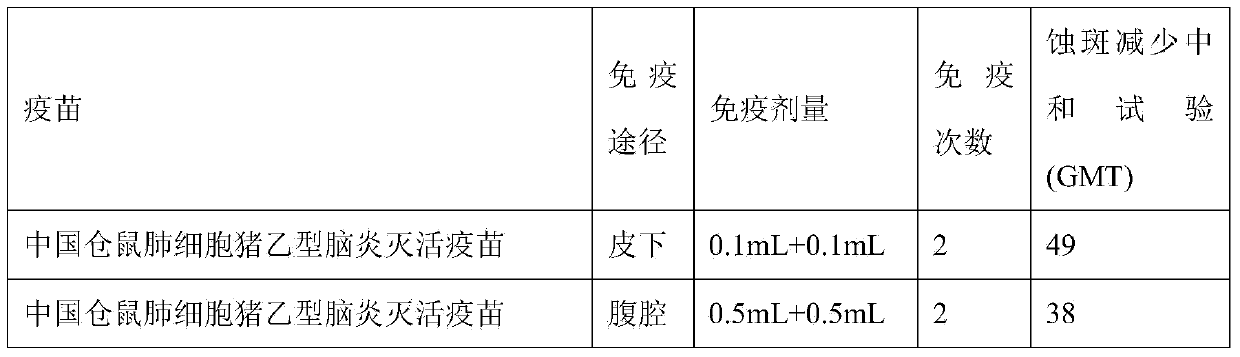
[0048] (2) Cell culture and inoculation: Chinese hamster lung cells (V79 strain) were passaged at a ratio of 1:2 to 1:6. When the cells grew into a dense monolayer, the Chinese hamster lung cells that had grown into a dense monolayer ( V79 strain) was washed twice with Hank's solution, then the virus seed diluted with serum-free RPMI 1640 was added, inoculated at an MOI of 0.1, and adsorbed and cultured for 30 min to 1 h, then replaced with RPMI 1640 containing 4% bovine serum, adding 0.05% Arginine and 200IU antibiotics, pH adjusted to 7.4-7.6, culture...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com