Composition for preventing hair loss and accelerating hair growth
A composition and compound technology, applied in the directions of drug combination, hair care, medical preparations containing active ingredients, etc., to achieve the effect of preventing hair loss and promoting hair growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~49
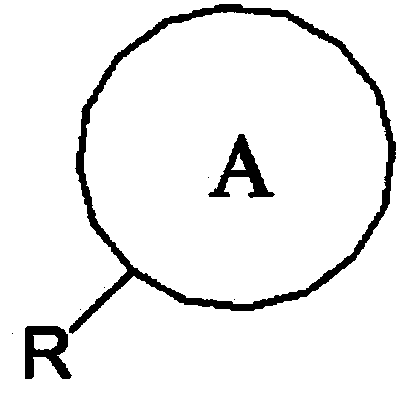
[0028] After commercially available Compound A (starting material, 10 mmol) was placed in a two-neck round bottom flask at room temperature, 50 ml of dichloromethane was introduced, and the mixture was frozen with an ice bath. 1 mmol of dimethylaminopyridine and 1.1 mmol of triethylamine were further introduced as catalysts, and then compound B (acyl halide, inducing substance, 1.2 mmol) was slowly added dropwise thereto. After the dropwise addition was completed, the mixture was allowed to react at room temperature. 100 ml of dilute hydrochloric acid solution was introduced into the reaction product, and extraction was performed using 200 ml of dichloromethane. After drying the dichloromethane layer under reduced pressure, separation and purification were performed using silica gel column chromatography (ethyl acetate:hexane=1:50), thereby obtaining a final product. The obtained product was identified by fast atom bombardment mass spectrometry (hereinafter written as "FAB-MS...
Embodiment 50~72
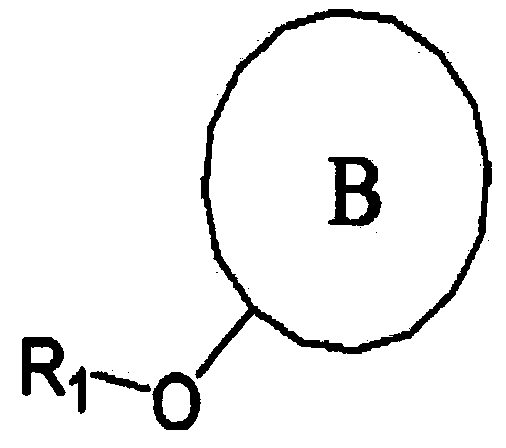
[0034] 1.60 g of sodium hydride (60%, 40 mmol) and 200 ml of anhydrous tetrahydrofuran were introduced into a 500 ml three-necked flask equipped with a thermostat and a condenser, and the mixture was stirred at room temperature. Compound C (starting material, 20 mmol) in solid state was then introduced into the mixture at room temperature, followed by stirring for 30 minutes. In addition, compound D (inducing substance, 22 mmol) was introduced into the mixture at room temperature, and stirred at tetrahydrofuran reflux temperature for 36 hours or more. After that, 100 ml of water was added to the reaction product, and extraction was performed using 200 ml of ether. The extracted product was dried using anhydrous magnesium sulfate, then filtered and concentrated. Thereafter, separation and purification were performed using silica gel column chromatography (ethyl acetate:hexane=1:60) to obtain a final product. The obtained product was identified by FAB-MS.
[0035] [Table 2] ...
experiment example 1-5
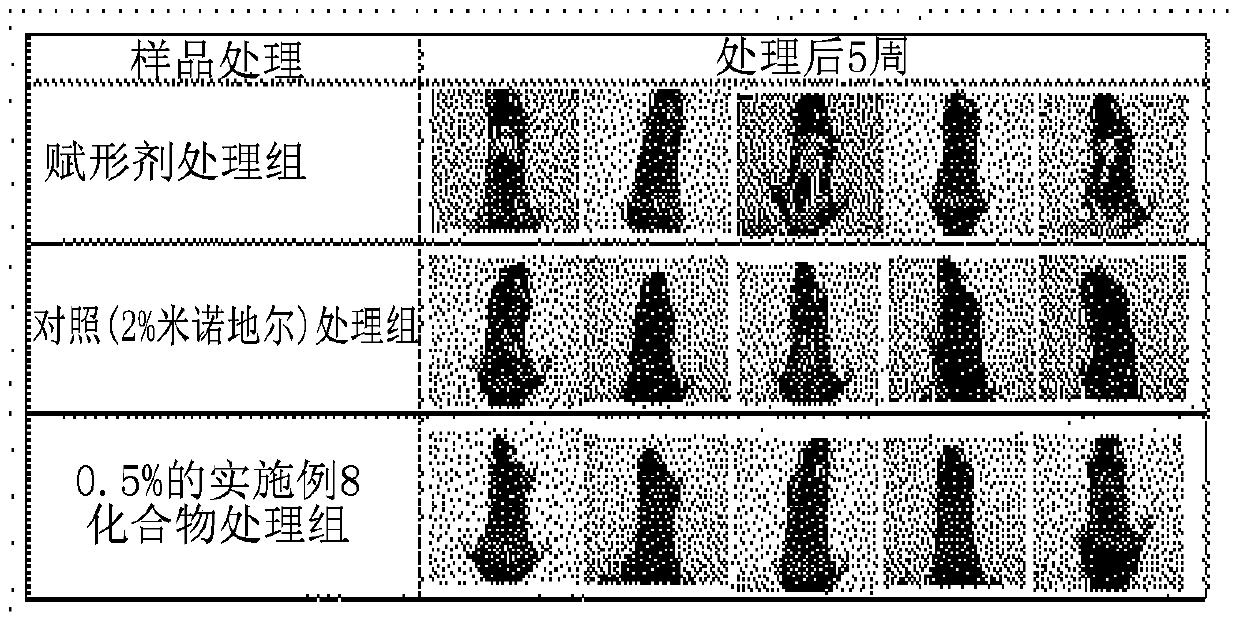
[0039] [Experimental Example 1] - Evaluation of 5α-reductase inhibitory efficacy
[0040] In 1ml of dermal papilla cells (DPCs) in 5x10 5 Inoculated into each well of a 24-well plate per ml, and then cultured for 24 hours, each reaction product prepared according to the procedure described in Examples 1 to 72 was added to a separate in the hole. After 1 hour, the wells were stimulated with 50 nM testosterone (dissolved in DMSO). After culturing for 24 hours, the supernatant was discarded, whereby a DPc pellet was obtained. The content of dihydrotestosterone (DHT) (DB52021, Hamburg, Germany) was determined by enzyme-linked immunosorbent assay. Inoculate 100 μL of the DPCs cell mass into each dihydrotestosterone (DHT) ELISA microtiter strip coated with rabbit anti-DHT antibody, inoculate 25 μL of DHT antiserum into each well, and inoculate at 2 ° C ~ 8 The reaction was carried out at ℃ for 15 hours to 20 hours, and then it was washed three times. After that, 100 μL of DHT-H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com