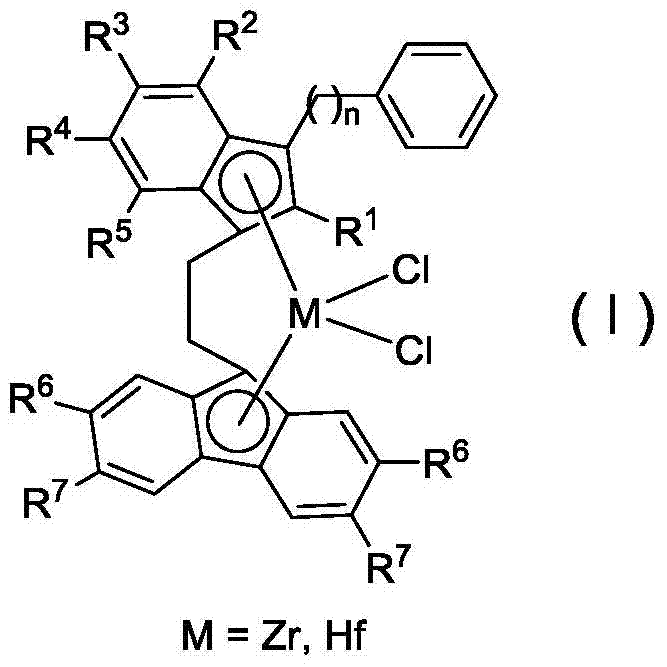
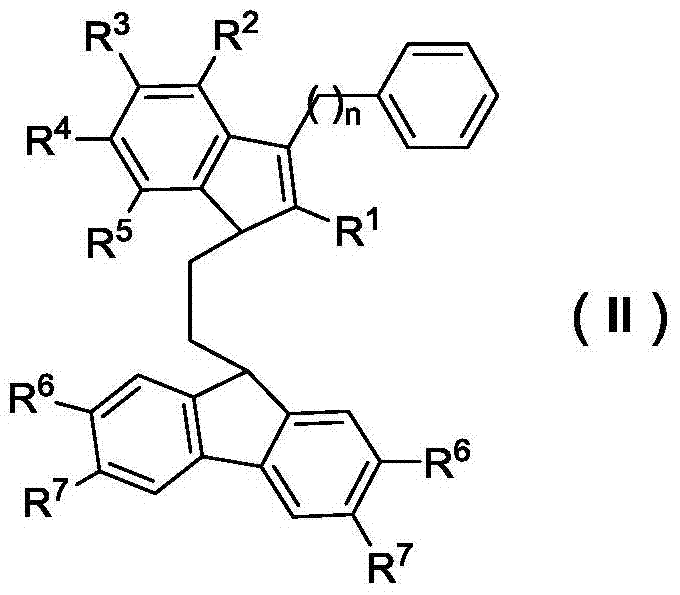
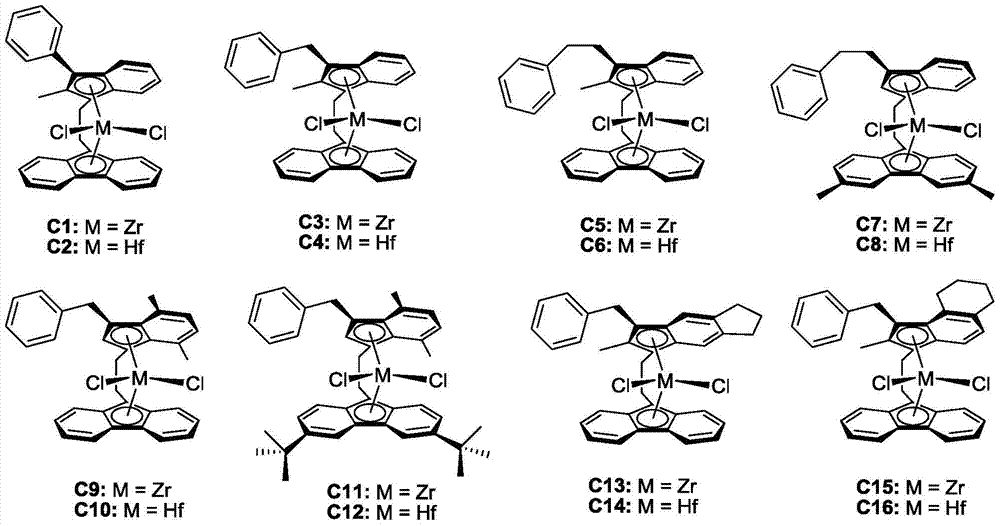
Bridged indene fluorene zirconium, hafnium compound and its preparation method and application in propylene oligomerization
A hafnium compound, the technology of catalyzing propylene, applied in chemical instruments and methods, organic chemistry, metallocene, etc., can solve the problems of limited molecular weight range of propylene oligomers, limited activity and selectivity, and limited practical value catalyst structure, etc., to achieve high catalytic performance active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of zirconium complex C1
[0045] (1) Synthesis of substituted indene In1
[0046]
[0047] Put 0.67g of magnesium bars in a three-necked flask, add 30mL of anhydrous ether, and dropwise add 4.10g of bromobenzene in 30mL of ether solution. At room temperature, 3.20 g of 2-methyl-2,3-dihydro-1-indanone in 40 mL of diethyl ether solution was added dropwise, and the reaction was stirred at room temperature for 3 h. In an ice-water bath, use 30 mL of saturated NH 4 Cl aqueous solution was used to terminate the reaction, the aqueous phase was extracted with 120 mL ether, and the organic phase was extracted with anhydrous MgSO 4 Dry, spin dry, dissolve with 70mL toluene, add 0.50g p-toluenesulfonic acid monohydrate, reflux for 4h, add 30mL saturated NaHCO 3 , the aqueous phase was extracted with 100mL petroleum ether, and the organic phase was extracted with anhydrous MgSO 4 Dry, spin off the solvent, and separate by column to obtain 3.83 g of light yellow liq...
Embodiment 2
[0057] Synthesis of zirconium complex C3
[0058] (1) Synthesis of substituted indene In2
[0059]
[0060] 2.90g of 2-methylindene was placed in a Schlenk bottle, dissolved in 60mL of ether, and 10.1mL (2.2mol / L) of n-butyllithium in n-hexane was added dropwise in a liquid nitrogen-ethanol bath, and the reaction was stirred overnight. Add 3.81g benzyl bromide in 20mL diethyl ether solution under ice-cooling, stir at room temperature for 4d, add 30mL saturated NH 4 Cl aqueous solution was used to terminate the reaction, the aqueous phase was extracted with 45 mL ether, and the organic phase was extracted with anhydrous MgSO 4 After drying, the solvent was removed and separated by column to obtain 3.58 g of light yellow liquid with a yield of about 73.3%.
[0061] 1 H NMR (400MHz, 298K, CDCl 3 ):δ7.21-7.14(m,3H,Ar-H),7.14-7.05(m,4H,Ar-H),6.89(td,J=7.4,1.8Hz,1H,Ar-H),6.76( d,J=7.4Hz,1H,Ar-H),6.35(s,1H,3-Ind-CH),3.47(dd,J=8.6,5.6Hz,1H,1-Ind-CH),3.23(dd ,J=13.8,5.6Hz,1H,C...
Embodiment 3
[0070] Synthesis of zirconium complex C9
[0071] (1) Synthesis of substituted indene In5
[0072]
[0073] Put 3.40g of 4,7-dimethylindene in a Schlenk bottle, dissolve it with 60mL of ether, add 10.7mL (2.2mol / L) of n-butyl lithium in n-hexane dropwise in a liquid nitrogen-ethanol bath, and stir the reaction overnight. Add 4.04g of benzyl bromide in 20mL ether solution under ice-cooling, stir at room temperature for 4d, and dissolve with 30mL saturated NH 4 Cl aqueous solution was used to terminate the reaction, the aqueous phase was extracted with 45 mL ether, and the organic phase was extracted with anhydrous MgSO 4 After drying and removing the solvent, it was separated by column to obtain 3.66 g of light yellow liquid with a yield of about 66.2%.
[0074] 1 H NMR (400MHz, 298K, CDCl 3 ):δ7.38-7.19(m,5H,Ar-H),7.04-6.90(m,2H,Ar-H),6.85(d,J=5.6Hz,1H,3-Ind-CH),6.37( d,J=5.6Hz,1H,2-Ind-CH),3.82-3.72(m,1H,1-Ind-CH),3.64(dd,J=13.2,4.4Hz,1H,CH 2 ),2.54(s,3H,CH 3 ),2.39...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com