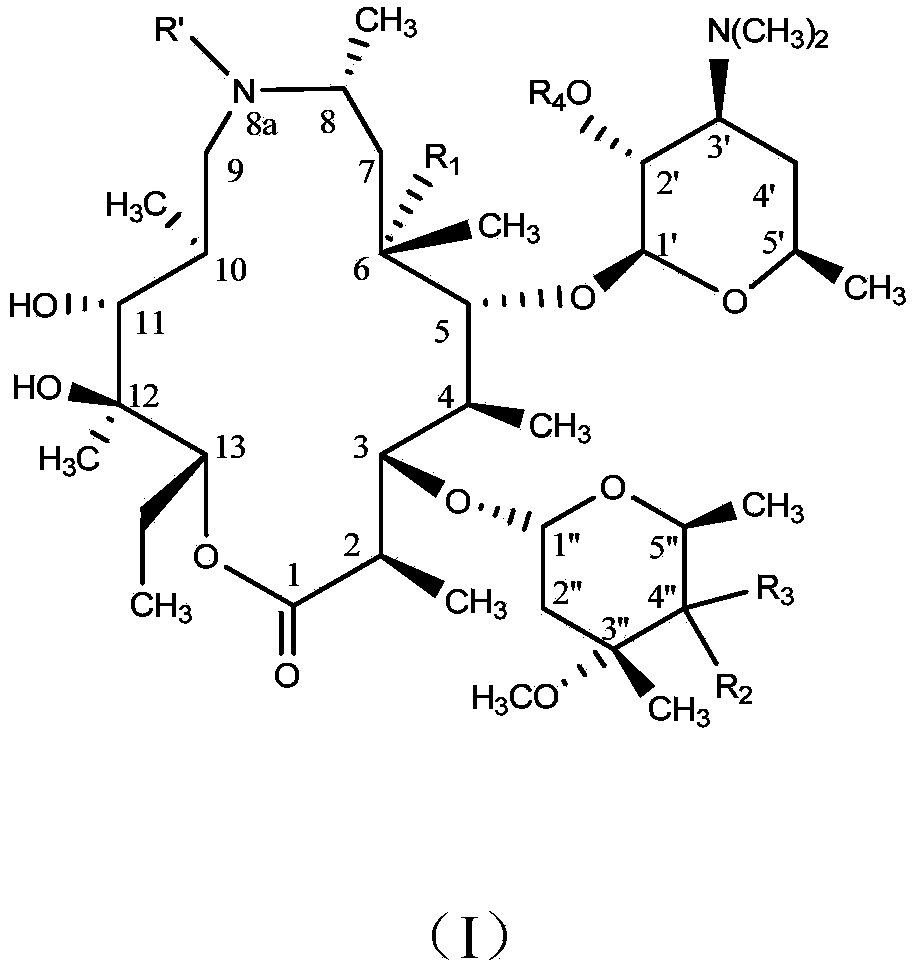
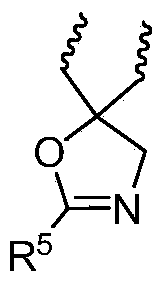
Macrolide compound and preparation method and application thereof
A compound and composition technology, applied in the field of macrolide compounds, can solve problems such as clinical treatment difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Open the low-temperature tank and set the temperature to -12°C; add 500mL of dichloromethane into a 1.0L three-neck flask with a 1L graduated cylinder, place it in the low-temperature tank and stir to cool down, and use a tray balance to take 50g (0.068mol) of compound 2 into the reaction flask Add 300mL of dichloromethane into the reaction flask with a 1L graduated cylinder, stir to dissolve, and cool down; cool to an internal temperature of 0-5°C, keep the temperature of the reaction solution at 0-5°C, and slowly add 11.98mL of benzyl chloroformate and dichloroformate A mixed solution of 60mL of methane was added, and the reaction was incubated for 1 hour; the progress of the reaction was monitored by thin-layer chromatography (developing solvent: dichloromethane / methanol=10:1, and two drops of ammonia water were added). After the reaction is completed, the reaction solution is concentrated under reduced pressure at a temperature ≤ 50°C and a vacuum degree ≤ -0.086Mpa ...
Embodiment 2
[0120] Turn on the low-temperature refrigeration equipment, adjust the temperature to -75°C, and lower the temperature to -70~-60°C; transfer about 300mL of compound 3 liquid obtained from the concentration of Example 1 to a 1.0L three-necked reaction flask, and store at room temperature (25~30°C) Add 106.46 mL (117.11 g, 1.498 mol) of dimethyl sulfoxide; after the addition of dimethyl sulfoxide, place the reaction bottle in a low-temperature bath and stir to cool down to -70~-60 °C, and take 21.55 mL of trifluoroacetic anhydride ( 0.152mol, 31.865g) to control the rate of addition to maintain the temperature of the reaction solution at -65~-60°C, slowly drop it, and keep it warm for 0.5 hours; then keep the temperature of the reaction solution at -65~-60°C and slowly drop triethylamine 47.3mL (0.339mol, 34.35g), after dripping, keep stirring for 0.5 hours; after the reaction, the reaction solution rises to room temperature. Transfer the reaction solution raised to room temper...
Embodiment 3
[0124] Add 50g (0.0456mol) of the compound 4 obtained in Example 2 and 80ml of dichloromethane into a 3.0L beaker. After stirring and mixing, the solution is dried with 15g of anhydrous magnesium sulfate for 20min, filtered with suction, and the filtrate is then washed with 7g of anhydrous magnesium sulfate. Secondary drying for 20 minutes, suction filtration, filter residue washed with dichloromethane, dichloromethane added to the filtrate to 160ml, (the water content of the filtrate should be less than 0.3%) for later use. Turn on the low-temperature refrigeration equipment, and adjust the temperature to -8°C; add 170 mL of tetrahydrofuran (dried over anhydrous magnesium sulfate for 30 minutes, and suction filter) into a 1.0L three-necked bottle, place it in a low-temperature tank and stir to cool down to -5-0°C, Add 20 g (0.1273 mol) of trimethylsulfur bromide, add 20 g (0.1786 mol) of potassium tert-butoxide under temperature control, use a circulating water pump to evacuat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com