Use of compound MEAN in preparation of medicine for inhibiting HCV replication
A technology of compounds and drugs, applied in the field of medicine, can solve problems such as unreported antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
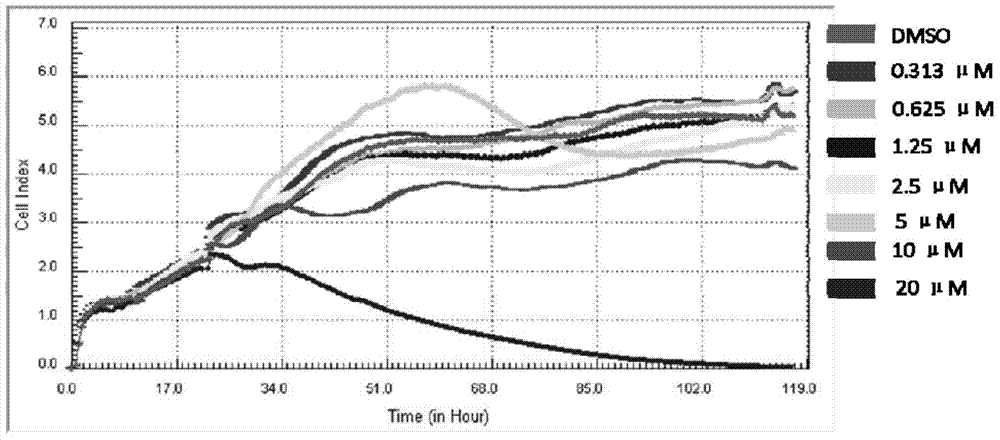
[0027] Embodiment 1 drug cytotoxicity analysis
[0028] cell culture
[0029] Huh-7.5.1 cells were cultured in DMEM medium containing 10% fetal bovine serum in an incubator at 37°C and 5% CO2 saturated humidity. 100U / ml of penicillin and 100ug / ml of streptomycin were added to the culture medium.
[0030] Preparation of HCV RNA Transcripts
[0031] Take 15 μg of pFL-JC1 plasmid, carry out enzyme digestion reaction with restriction endonuclease XbaI, incubate at 37°C for 2 hours, and then check whether it is completely linearized by agarose gel electrophoresis. The digested products were digested with mung bean nuclease and proteinase K, and then the DNA was extracted and purified by the phenol-chloroform method, and then detected and quantified by agarose gel electrophoresis. Then 2 μg of DNA template was taken, and T7 in vitro transcription kit was used for in vitro transcription according to the instructions. The obtained RNA transcripts were detected by agarose gel elect...
Embodiment 2
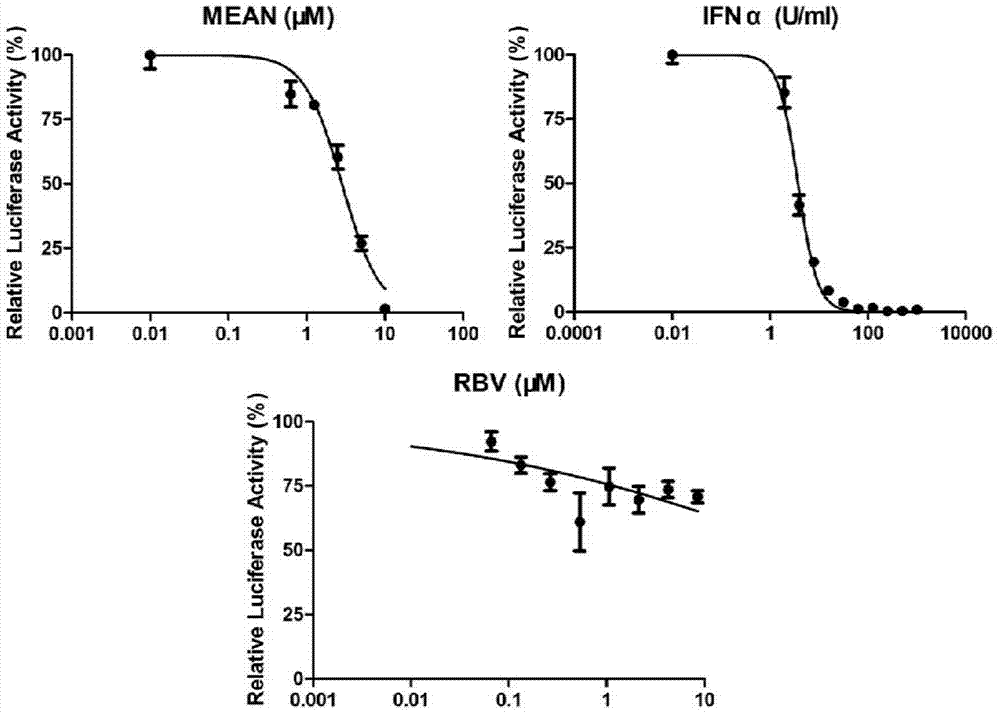
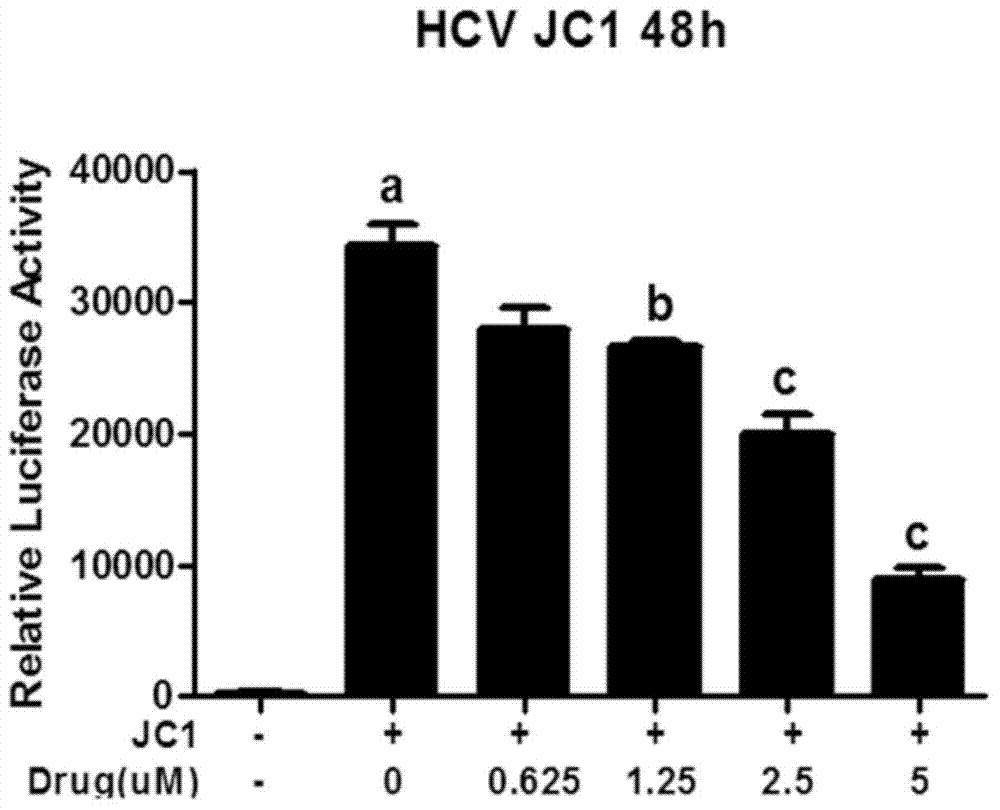
[0037] Embodiment 2 MEAN anti-HCV efficacy analysis
[0038] Analysis of anti-HCV effect in vitro
[0039] Huh7.5.1 cells were inoculated at 5000 per well in a 96-well plate and incubated overnight. The wild-type virus JC1-luc was used to infect the cells at MOI0.01 for 24 hours, and then given 0.625 μM, 1.25 μM, 2.5 μM, 5 μM MEAN, and 48 hours later, micro Luciferase activity was quantified with a plate luminometer (Turner Biosystems). The inhibitory activity of MEAN was verified by monitoring the expression levels of HCV RNA and protein in Huh7.5.1 cells infected with the virus.
[0040] Fluorescent quantitative PCR
[0041] Fluorescent quantitative PCR was used to detect the relative amount of HCV RNA in the cells. Cells were collected after trypsinization, and Trizol was added to each tube to extract total RNA according to the operating instructions, and the concentration was measured by an ultraviolet spectrophotometer. Take 4 μl of RNA and remove the genomic DNA acco...
Embodiment 3
[0048] Embodiment 3 MEAN anti-E2 mutant strain JC1 / N415D or JC1 / G451R effect
[0049] Anti-E2 mutant strain JC1 / N415D or JC1 / G451R effect analysis in vitro
[0050] Huh7.5.1 cells were inoculated in 96-well plates at 5000 per well and incubated overnight. The cells were infected with the mutant virus JC1 / N415D or JC1 / G451R at MOI0.01 for 24 hours, and MEAN was given at a concentration gradient. After 48 hours, a microplate luminometer was used to (Turner Biosystems) to quantify luciferase activity.
[0051] Such as Figure 5 As shown, MEAN can also inhibit the replication of mutant JC1 / N415D and JC1 / G451R viruses.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com