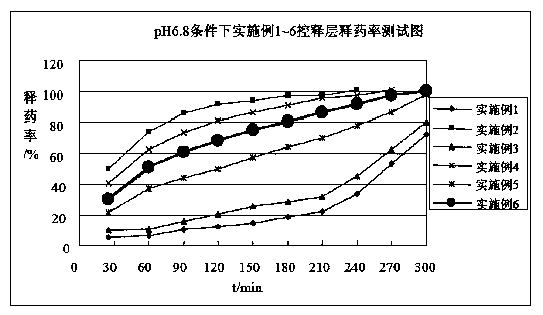
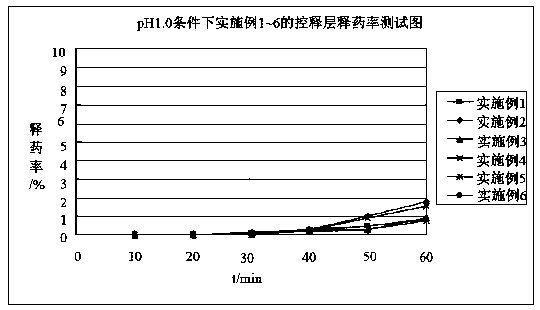
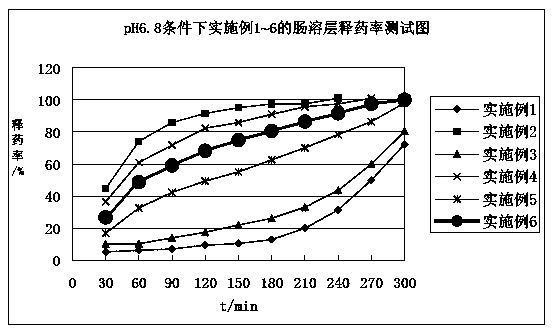
Diammonium glycyrrhizinate enteric-coated sustained-release pellet and preparation method thereof
A technology of diammonium glycyrrhizinate and sustained-release pellets, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, block delivery, etc., and can solve the problem that ammonium glycyrrhizinate pellets cannot control the release position and patients It is easy to produce adverse reactions, and the release rate curve changes quickly, etc., to achieve the effect of increasing adhesion and penetration, improving bioavailability, and facilitating dispersion and absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The diammonium glycyrrhizinate enteric-coated sustained-release pellets of the present embodiment include a ball core, and a controlled-release layer and an enteric-coated layer wrapped on the outside of the ball core in sequence from the inside to the outside; The layer and the enteric layer are configured according to the weight ratio of 98:1:1;
[0036] The ball core is composed of the following ingredients in parts by weight: 35 parts of diammonium glycyrrhizinate, 0.5 part of lecithin, 0.5 part of chenodeoxycholic acid, and 0.5 part of microcrystalline cellulose;
[0037] The controlled-release layer is composed of the following ingredients in parts by weight: 93 parts of ethyl cellulose, 6 parts of albumin, and 6 parts of alanine;
[0038] The enteric layer is composed of the following components in parts by weight: 90 parts of methacrylate copolymer, 6 parts of lecithin, 0.9 parts of sodium hydroxide, and 17 parts of magnesium stearate.
[0039] The preparation ...
Embodiment 2
[0044] The diammonium glycyrrhizinate enteric-coated sustained-release pellets of the present embodiment include a ball core, and a controlled-release layer and an enteric-coated layer wrapped on the outside of the ball core in sequence from the inside to the outside; The layer and the enteric layer are configured in a weight ratio of 98: 5: 5;
[0045] The pellet core is composed of the following ingredients in parts by weight: 35 parts of diammonium glycyrrhizinate, 5 parts of lecithin, 5 parts of chenodeoxycholic acid, and 40 parts of microcrystalline cellulose;
[0046] The controlled-release layer is composed of the following ingredients in parts by weight: 88 parts of ethyl cellulose, 6 parts of albumin, and 6 parts of cysteine;
[0047] The enteric layer is composed of the following ingredients in parts by weight: 90 parts of methacrylate copolymer, 6 parts of lecithin, 0.2 parts of sodium hydroxide, and 22 parts of magnesium stearate.
[0048] The preparation method c...
Embodiment 3
[0053] The diammonium glycyrrhizinate enteric-coated sustained-release pellets of the present embodiment include a ball core, and a controlled-release layer and an enteric-coated layer wrapped on the outside of the ball core in sequence from the inside to the outside; The layer and the enteric layer are configured according to the weight ratio of 80:1:1;
[0054] The ball core is composed of the following ingredients in parts by weight: 35 parts of diammonium glycyrrhizinate, 0.5 part of lecithin, 0.5 part of sodium glycocholate, and 0.5 part of microcrystalline cellulose;
[0055] The controlled-release layer is composed of the following ingredients in parts by weight: 93 parts of ethyl cellulose, 6 parts of albumin, and 6 parts of alanine;
[0056] The enteric layer is composed of the following components in parts by weight: 90 parts of methacrylate copolymer, 6 parts of lecithin, 0.9 parts of sodium hydroxide, and 17 parts of magnesium stearate.
[0057] The preparation me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com