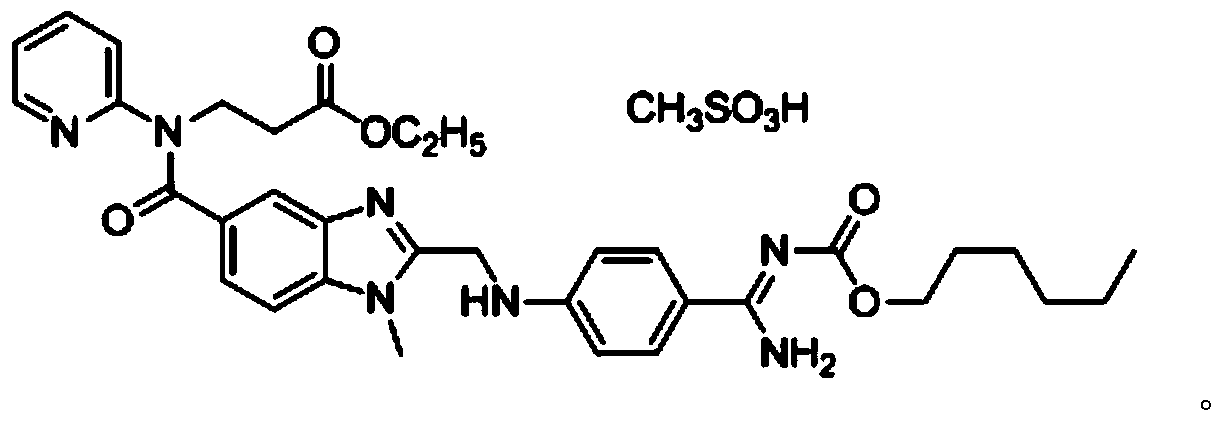
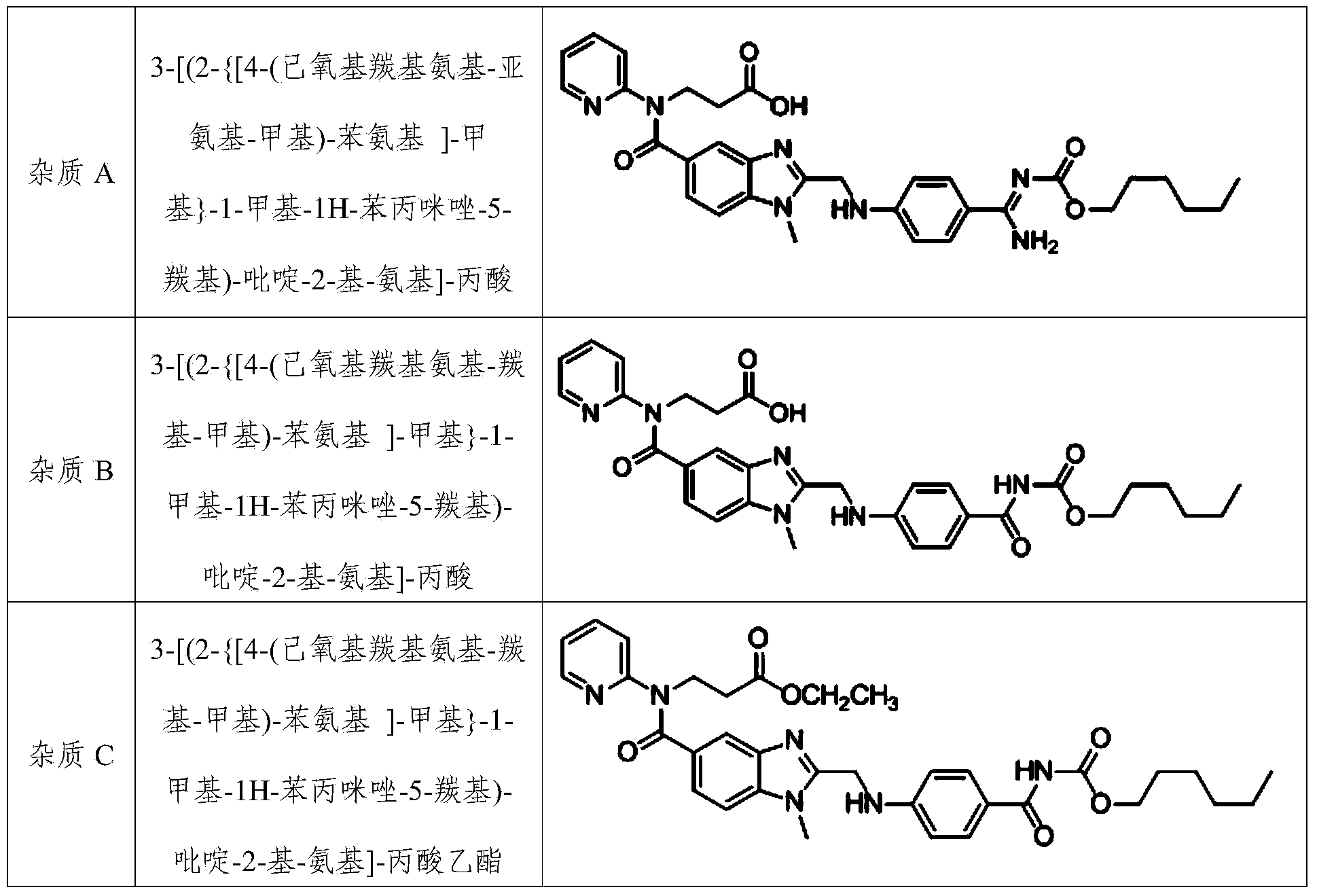
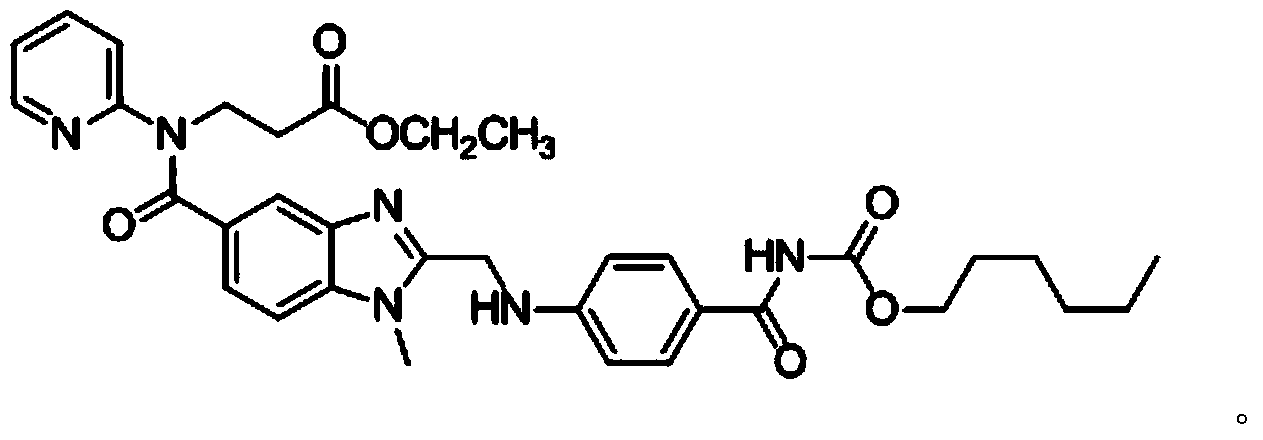
Method for preparing dabigatran etexilate hydrolysis impurity
A technology of dabigatran etexilate and impurities, applied in the field of preparation of dabigatran etexilate hydrolyzed impurities, to achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Prepare dabigatran etexilate hydrolysis impurities according to the following steps:
[0046] (1) In a 100ml single-necked flask, sequentially add 2g of dabigatran etexilate, 30ml of absolute ethanol, and 30ml of 0.5mol / L hydrochloric acid, heat to 40°C, and stir for 20h;
[0047] (2) The reaction solution obtained in step (1) is distilled under reduced pressure until oily matter is produced, the solvent is removed, 60ml of dichloromethane is added for extraction, and the extract is collected;
[0048] (3) separate and purify step (2) gained extract with medium pressure preparative chromatography, mobile phase is the aqueous solution of the acetonitrile of volume ratio 60:40 and pH4.4, and described aqueous solution is the ammonium acetate aqueous solution of concentration 0.2%, uses Acetic acid was used to adjust the pH value to 4.4, and the stationary phase was octadecyl silica gel. The components with a retention time of 17 to 20 minutes were collected under the cond...
Embodiment 2
[0052] Prepare dabigatran etexilate hydrolysis impurities according to the following steps:
[0053] (1) In a 100ml single-necked flask, sequentially add 2g of dabigatran etexilate mesylate, 50ml of anhydrous acetonitrile, and 30ml of 0.02mol / L sulfuric acid, heat to 50°C, and stir for 8 hours;
[0054] (2) The reaction solution obtained in step (1) is distilled under reduced pressure until oily matter is produced, the solvent is removed, 50ml of dichloromethane is added for extraction, and the extract is collected;
[0055] (3) separate and purify the extract obtained in step (2) with medium pressure preparative chromatography, mobile phase is the aqueous solution of the acetonitrile of volume ratio 55:40 and pH 4.0, and described aqueous solution is the ammonium acetate aqueous solution of concentration 0.3%, with acetic acid Adjust its pH value to 4.0, use octadecyl silica gel as the stationary phase, collect components with a retention time of 17-20 min at a detection wave...
Embodiment 3
[0058] Prepare dabigatran etexilate hydrolysis impurities according to the following steps:
[0059] (1) In a 100ml single-necked flask, add 2g of dabigatran etexilate, 30ml of anhydrous isopropanol, and 30ml of 2mol / L p-toluenesulfonic acid successively, heat to 10°C, and stir for 36h;
[0060] (2) The reaction solution obtained in step (1) is distilled under reduced pressure until oily matter is produced, the solvent is removed, 40ml of dichloromethane is added for extraction, and the extract is collected;
[0061] (3) separate and purify step (2) gained extract with medium pressure preparative chromatography, mobile phase is the aqueous solution of the acetonitrile of volume ratio 60:40 and pH 5.0, and described aqueous solution is the ammonium acetate aqueous solution of concentration 0.1%, with acetic acid Adjust its pH value to 5.0, the stationary phase is octadecyl silica gel, collect the components with a retention time of 17 to 20 min at a detection wavelength of 315 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com