A kind of method that uses fly ash to prepare aluminum-magnesium alloy
An aluminum-magnesium alloy and fly ash technology, applied in the field of non-ferrous metallurgy, can solve the problems of no industrial application, easy generation of dendrites, low electrolysis temperature, etc., to solve the problem of dendrites generated by electrolysis, easy equipment, and low electrolysis temperature. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
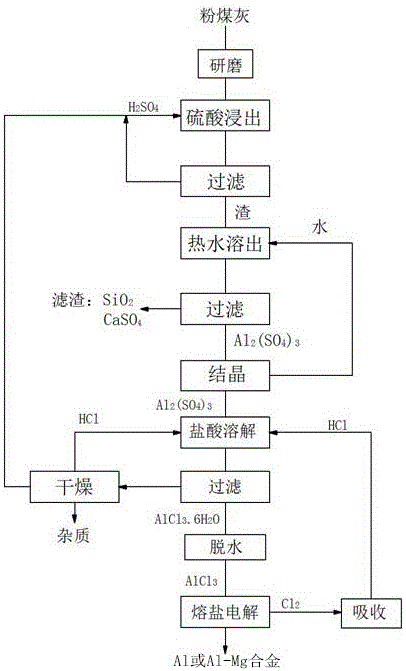
[0026] (1) Fly ash acid leaching to produce aluminum chloride: grind the fly ash to 100-200 μm, mix it with sulfuric acid according to the mass ratio of 1: 4.5 for leaching reaction, filter, react at 280°C for 3 hours, add to the filter residue Water with 3 times the mass is boiled at 65°C for 60 minutes to dissolve the reactant, filter to remove the residue, and obtain an aluminum sulfate solution. Evaporate and crystallize the aluminum sulfate solution, mix it with hydrochloric acid with a mass concentration of 38%, and then inject HCl gas , to precipitate AlCl 3 ·6H 2 O crystals, dried and dehydrated at 110°C to produce anhydrous AlCl 3 ;
[0027] (2) Electrolysis of aluminum chloride to produce aluminum-magnesium alloy: graphite is used as a bipolar electrode with a pole distance of 1 cm. The electrolyte system used is composed of flux, melt and additives. The composition of the flux is: NaCl: 23.7 %, KCl: 33.5%, MgCl 2 : 42.8%, then add molten AlCl that accounts for 5...
Embodiment 2
[0029] (1) Fly ash acid leaching to produce aluminum chloride: Grind fly ash to 100-200 μm, mix with sulfuric acid at a mass ratio of 1:10, filter, react at 300°C for 2.8 hours, pour into the filter residue Add 4 times the mass of water, boil and dissolve at 75°C for 50 minutes, dissolve the reactant, filter to remove the residue, and obtain an aluminum sulfate solution, concentrate the aluminum sulfate solution to a density of 1.4g / mL, and mix with 25% hydrochloric acid Mix, then pass HCl gas, precipitate AlCl 3 ·6H 2 O crystals, dried and dehydrated at 120°C to produce anhydrous AlCl 3 ;
[0030] (2) Electrolysis of aluminum chloride to produce aluminum-magnesium alloy: silicon carbide is used as a bipolar electrode with a pole distance of 1.5cm. The electrolyte system used is composed of flux, melt and additives. The flux is: NaCl: 39.6% by mass %, KCl: 26.5%, MgCl 2 :33.9%, then add molten AlCl that accounts for 10% of flux mass 3 , the additive KF accounting for 5% o...
Embodiment 3
[0032] (1) Fly ash acid leaching to produce aluminum chloride: Grind fly ash to 100-200 μm, mix with sulfuric acid at a mass ratio of 1:6 for leaching reaction, filter, react at 350°C for 2.5h, pour into filter residue Add 5 times the mass of water, boil and dissolve at 80°C for 45 minutes, dissolve the reactant, filter to remove the residue, and obtain an aluminum sulfate solution, concentrate the aluminum sulfate solution to a density of 1.4g / mL, and mix with hydrochloric acid with a mass concentration of 18% Mix, then pass HCl gas, precipitate AlCl 3 ·6H 2 O crystals, dried and dehydrated at 130°C to produce anhydrous AlCl 3 ;
[0033] (2) Electrolysis of aluminum chloride to produce aluminum-magnesium alloy: graphite is used as a bipolar electrode with a pole distance of 2.5 cm. The electrolyte system used is composed of flux, melt and additives. The composition of the flux is: KCl: 67.0%, MgCl 2 : 33.0%, then add molten AlCl that accounts for 20% of the flux mass 3 ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com