Granule containing acrylic acid resin and desloratadine, and preparation method thereof
A desloratadine and acrylic resin technology, applied in the field of medicine, can solve the problems of elevated related substances, unsuitable desloratadine granules, inability to achieve taste masking and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
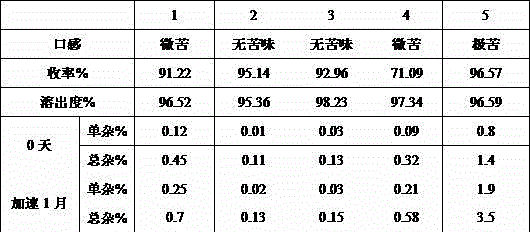
[0012] In this embodiment, the ratio of desloratadine to acrylic resin is 1:1, the ratio of acrylic resin and desloratadine solid to organic solvent is 1:2, and the selected organic solvent is ethanol. The distribution ratio for each group is:
[0013] components percentage(%) Desloratadine 0.5 Acrylic 0.5 Mannitol 20 sucrose 65 microcrystalline cellulose 9.0 Povidone K30 1.5 Magnesium stearate 1.0 sunset yellow 0.5 xanthan gum 1.0 Sweet Orange Flavor 1.0 Total 100
[0014] Preparation process: Add 5g of acrylic resin and 5g of desloratadine into 20g of ethanol and stir for 30 minutes to fully disperse and form a complex. The clathrate solution is dried at 50°C by spray drying method , collect the clathrate granules after drying, then add 200g mannitol, 650g sucrose, 90g microcrystalline cellulose, with 10% povidone K30, adopt wet granulation process granulation as binder, wet granules are again i...
Embodiment 2
[0016] In this embodiment, the ratio of desloratadine to acrylic resin is 1:10, and the ratio of acrylic resin to desloratadine solid to ethanol is 1:10. The distribution ratio for each group is:
[0017] components percentage(%) Desloratadine Hydrochloride 0.5 Acrylic 5 Mannitol 20 sucrose 60 microcrystalline cellulose 9.0 hypromellose 1.5 Magnesium stearate 1.0 lemon yellow 0.5 Sodium carboxymethyl cellulose 1.0 apple flavor 1.0 Total 100
[0018] Preparation process: Add 50g of acrylic resin and 5g of desloratadine into 550g of ethanol and stir for 30 minutes to fully disperse and form a complex. The clathrate solution is dried at 70°C by spray drying , collect the clathrate particles after drying, then add 200g mannitol, 600g sucrose, 90g microcrystalline cellulose, use 10% hypromellose as a binder to granulate by wet granulation process, and then wet the granules in Dry at 50°C, the solid co...
Embodiment 3
[0020] In this embodiment, the ratio of desloratadine to acrylic resin is 1:5, and the ratio of acrylic resin to desloratadine solid to ethanol is 1:5. The distribution ratio for each group is:
[0021] components percentage(%) Desloratadine Hydrochloride 0.5 Acrylic 2.5 Mannitol 20 sucrose 60 microcrystalline cellulose 11.5 Magnesium stearate 1.0 lemon yellow 0.5 Sodium carboxymethyl cellulose 1.0 apple flavor 1.0 Total 100
[0022] Preparation process: Add 25g of acrylic resin and 5g of desloratadine into 150% ethanol and stir for 30 minutes to fully disperse and form a complex. The clathrate solution is dried at 60°C by spray drying , collect the clathrate granules after drying, then add 200g mannitol, 600g sucrose, 10g microcrystalline cellulose, adopt dry granulation process to granulate, finally add 10g magnesium stearate, 5g tartrazine, 10g carboxymethyl cellulose Su sodium, 10g of apple flavor,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com