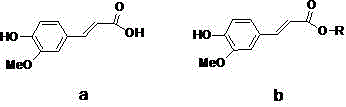Method for synthesis of ferulic acid ester by solid acid catalysis
A technology of solid acid catalysis and ferulate ester, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid esters, etc., can solve the problems of less research on catalytic synthesis, and achieve easy separation, high catalytic activity, non-corrosive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (Preparation of cyclohexyl ferulate)
[0022] Take 56.8g (0.2 mol) sodium metasilicate nonahydrate to make a 5.0% aqueous solution, start stirring, heat to 55°C, add 18mL (0.18mol) ethyl acetate dropwise, after the gel is formed, add dilute hydrochloric acid under stirring Adjust the pH value to about 4 and stop stirring. Suction filtration, the filter cake was washed with distilled water until the filtrate did not contain Cl - So far, vacuum drying at low temperature yielded 11.2 g of white silica powder.
[0023] Place the silica powder prepared above in a muffle furnace at 200°C for activation for 2 hours, take 10 g of the activated silica and mix it into 30 mL of 10% sodium bisulfate aqueous solution, soak for 24 hours, then suction filter, wash with distilled water until the filtrate is free of After being dried in vacuum at low temperature, it was activated in a muffle furnace at 200°C for 3 hours, cooled in a desiccator, and set aside.
[0024] In a 500mL thre...
Embodiment 2
[0026] (Preparation of n-pentyl ferulate)
[0027] Take 56.8g (0.2 mol) of sodium metasilicate nonahydrate to make a 5.0% aqueous solution, start stirring, heat to 50°C, add 18mL (0.18mol) of ethyl acetate dropwise, after the gel is formed, wash with dilute hydrochloric acid under stirring Adjust the pH value to about 4 and stop stirring. Suction filtration, the filter cake was washed with distilled water until the filtrate did not contain Cl - So far, vacuum drying at low temperature yielded 11.2 g of white silica powder.
[0028] Place the silica powder prepared above in a muffle furnace at 200°C for activation for 2 hours, take 10 g of the activated silica and mix it into 30 mL of 10% sodium bisulfate aqueous solution, soak for 24 hours, then suction filter, wash with distilled water until the filtrate is free of After being dried in vacuum at low temperature, it was activated in a muffle furnace at 200°C for 3 hours, cooled in a desiccator, and set aside.
[0029] I...
Embodiment 3
[0031] (Preparation of n-pentyl ferulate, catalyst recycling experiment)
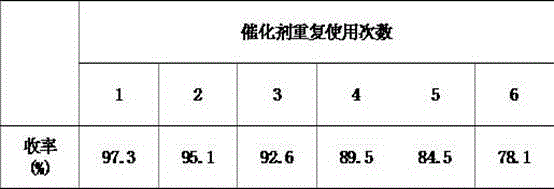
[0032] On the basis of keeping the same experimental conditions as in Example 2, the reusability of the catalyst was investigated, and the results are shown in Table 1.
[0033] Table 1 Effect of catalyst reuse on esterification reaction
[0034]
[0035] Through the data in the table can be analyzed, the prepared NaHSO 4 / SiO 2 Catalyst, in the esterification reaction of ferulic acid and n-amyl alcohol, when reused 5 times, still can guarantee that yield is greater than 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com