Process for production of chloroethylene by acetylene and dichloroethane catalytic reforming
A technology of dichloroethane and catalytic reforming, which is applied in the fields of organic chemistry, dehydrohalogenation preparation, hydrogen halide addition preparation, etc., can solve the problems of increased cost and many process equipment, and achieves increased inert gas content and improved reaction Efficiency, the effect of increasing investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
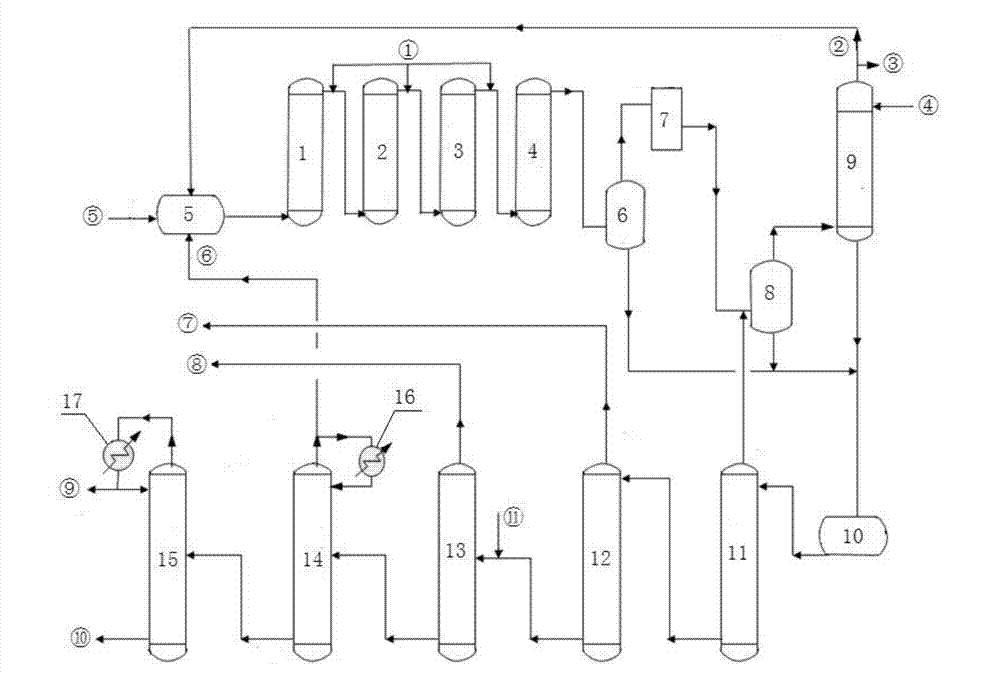
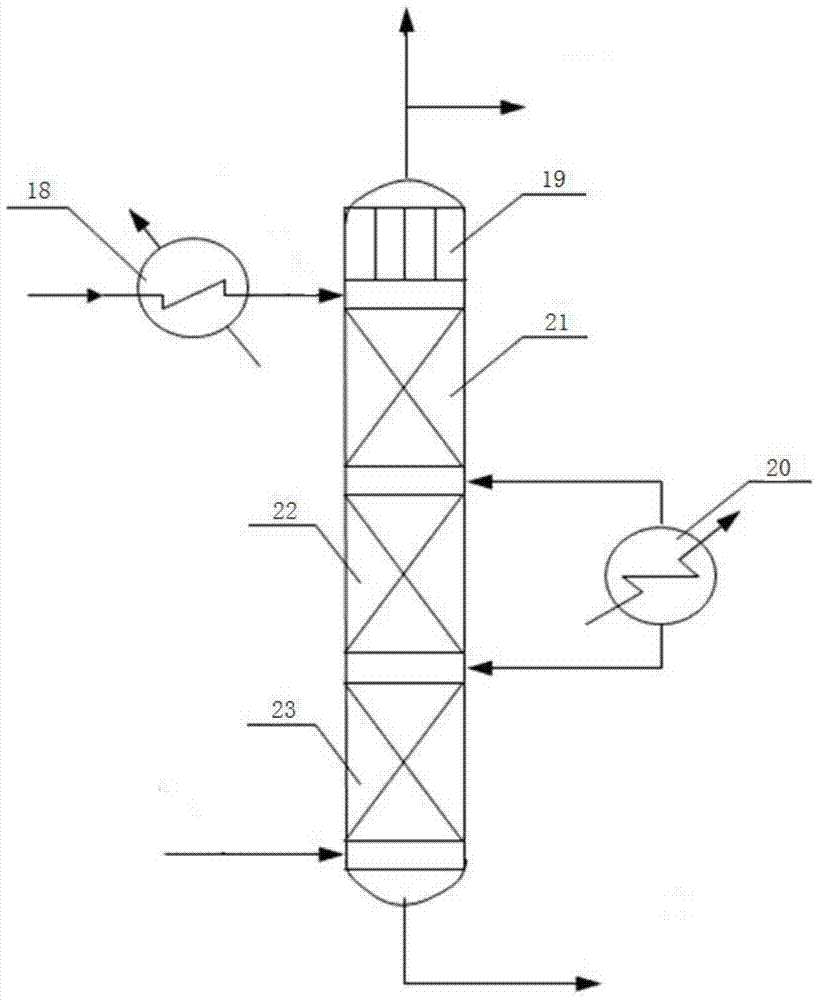
[0075] Gas phase dichloroethane (purity: wt=99.93%) from the dichloroethane rectifying tower top and raw material acetylene and the recycle gas (weight composition (wt %) from the spray tower top: vinyl chloride=1.94% , ethylene dichloride=3.83%, acetylene=49.06%, hydrogen chloride=19.45%, nitrogen oxide=25.71%; impurity 0.01%) by weight 83.36%: 10.55%: 6.09% after mixing in the gas mixing tank, by total The flow rate is 26398.23kg / h (the mixed gas volume per ton of catalyst is 4.224 tons per day), and it enters the 5-stage chilled fixed-bed reactor (inlet temperature 188°C, outlet temperature 230°C, with activated carbon-supported barium chloride catalyst inside, The pressure of each stage is controlled at 0.8MPa); liquid dichloroethane (temperature 25°C) is used to perform a cold shock between the two stages of reaction.
[0076] Reaction product (flow rate: 31917.54kg / h, weight composition (wt%): vinyl chloride=39.85%, dichloroethane=54.71%, acetylene=2.75%, hydrogen chlori...
Embodiment 2
[0081] Gas phase dichloroethane (purity: wt=99.93%) from dichloroethane rectifying tower top and raw material acetylene and the recycle gas (weight composition (wt %) from spray tower top: vinyl chloride=2.99% , ethylene dichloride=2.64%, acetylene=41.96%, hydrogen chloride=19.75%, nitrogen oxide=32.66%;) After mixing in the gas mixing tank by weight ratio 80.46:10.42:9.12, according to the total flow rate of 30486.84kg / h Processing speed (4.878 tons of mixed gas per ton of catalyst per day), enter the 5-stage chilled fixed-bed reactor (inlet temperature 195°C, outlet temperature 234°C, built-in carbon-supported barium chloride catalyst, the pressure of each stage is controlled at 0.8 MPa); liquid dichloroethane (temperature 25° C.) was used to perform a cold shock between the two stages of the reaction.
[0082] Reaction product (flow rate: 34014.08kg / h, weight composition (wt%): vinyl chloride=42.92%, dichloroethane=48.53%, acetylene=3.61%, hydrogen chloride=1.70%, nitrogen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com