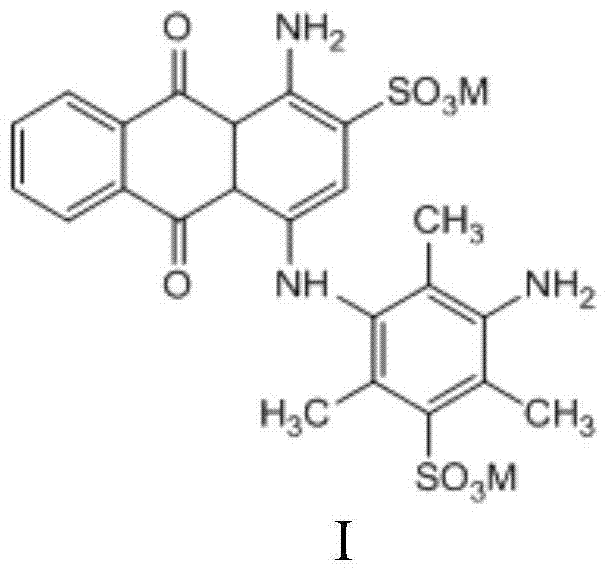
Preparation method of bromine ammonia blue
A technology of ammonium bromide and ammonium bromide, which is applied to the preparation of sulfonate, chemical instruments and methods, and anthracene dyes, can solve the problems of reducing the amount of catalyst used, incomplete conversion of bromamine, and reducing biscondensates, etc., to achieve Effects of reducing heavy metal content, increasing yield, and reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
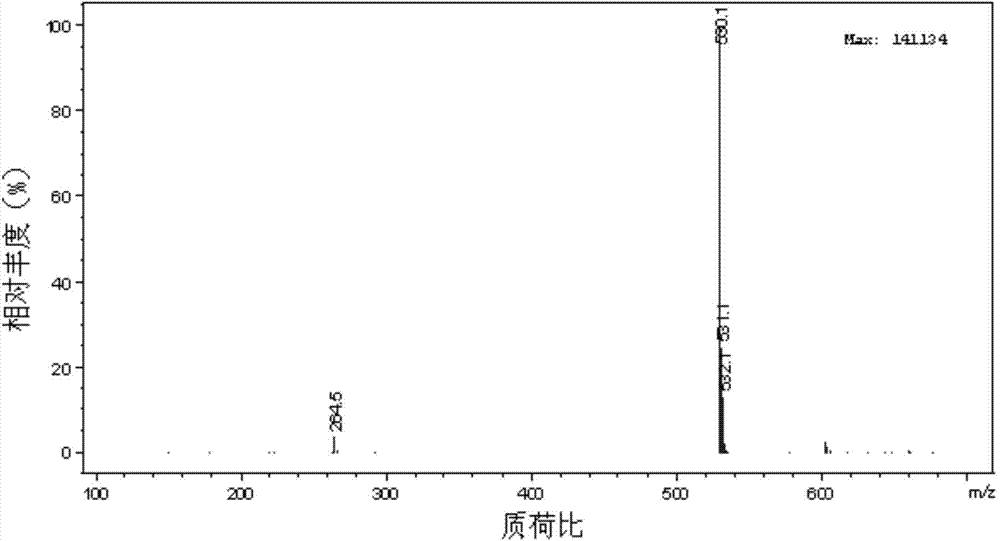
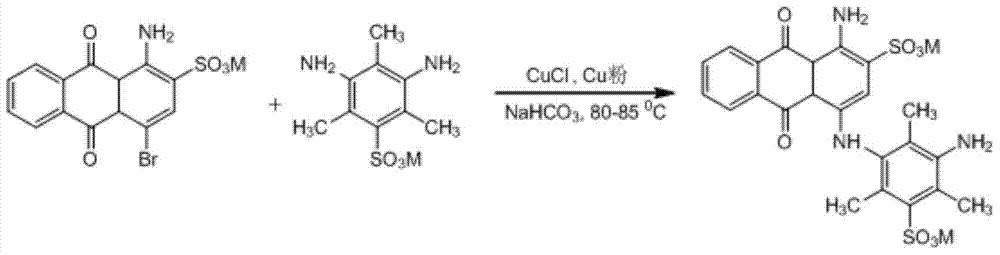
[0041] Take by weighing 9.20 grams of 2,4,6-trimethyl-3,5-diaminobenzenesulfonic acid (M acid), 8.4 grams of sodium bicarbonate, and 20 milliliters of water, add to the tank equipped with agitator, condenser and thermometer In a container, heat to 80°C with stirring. Weigh respectively 8.08 grams of bromine, 15% mol (based on bromine) of monovalent copper and 1,4,7,10-tetraazacyclododecane-1,3-diene (C 8 h 18 N 4 ) complex aqueous solution. Alternately add solid bromine and monovalent copper complexes into the container within 1 hour, keep the reaction temperature at 80-82°C, and the pH of the reaction system at 9, stir for three hours, keep the reaction temperature at 85-90°C, and stir the reaction , sampling for thin-layer chromatography and liquid chromatography analysis, no bromidine as the reaction end point. In the reaction liquid mixture, there are no hydrolyzed products of bromoacidic acid (purple side) and bicondensates, but there are traces of debrominated produc...
Embodiment 2
[0044] Weigh 23 grams of 2,4,6-trimethyl-3,5-diaminobenzenesulfonic acid (M acid), 26.5 grams of sodium carbonate, and 50 milliliters of water, and add them to a container equipped with a stirrer, a condenser and a thermometer , heated to 80°C with stirring. Weigh respectively 20.2 grams of bromine, 10% mol (based on bromine) of monovalent copper and 1,4,7,10-tetraazacyclododecane-1,3-diene (C 8 h 18 N 4 ) complex aqueous solution. Alternately add solid bromine and monovalent copper complexes into the container within 2.5 hours, keep the reaction temperature at 80-82°C, and the pH of the reaction system at 9, stir for three hours, keep the reaction temperature at 85-90°C, and stir the reaction , sampling for thin-layer chromatography and liquid chromatography analysis, no bromidine as the reaction end point. In the reaction liquid mixture, there are no hydrolyzed products of bromoacidic acid (purple side) and bicondensates, but there are traces of debrominated products.
...
Embodiment 3
[0047] Take by weighing 9.20 grams of 2,4,6-trimethyl-3,5-diaminobenzenesulfonic acid (M acid), 8.4 grams of sodium bicarbonate, and 20 milliliters of water, add to the tank equipped with agitator, condenser and thermometer In a container, heat to 80°C with stirring. Weigh respectively 8.08 grams of bromine, 10% mol (based on bromine) of monovalent copper and 1,4,7,10-tetraazacyclododecane-1,3-diene (C 8 h 18 N 4 ) complex ethanol solution. Alternately add solid bromine and monovalent copper complexes into the container within 1 hour, keep the reaction temperature at 80-82°C, the pH of the reaction system is 9, stir for 2 hours, keep the reaction temperature at 85-90°C, and stir the reaction , sampling for thin-layer chromatography and liquid chromatography analysis, no bromidine as the reaction end point. In the reaction liquid mixture, there are no hydrolyzed products of bromoacidic acid (purple side) and bicondensates, but there are traces of debrominated products.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com