R-type omega-aminotransferase and application thereof
A technology of transaminase and configuration, which is applied in the field of synthesis of chiral compounds and can solve the problems such as the inability to meet the needs of industrial production of chiral amines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
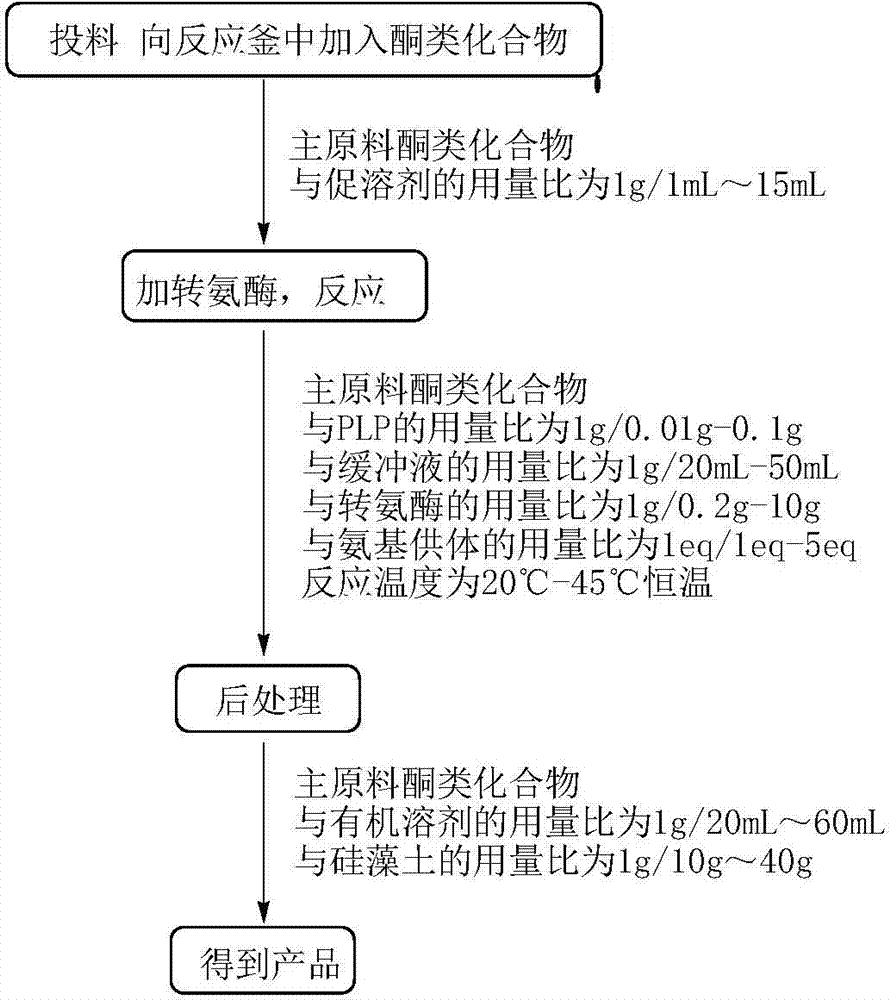
[0063] Embodiment 1: Preparation R type omega-transaminase
[0064] The concrete steps of the preparation method of R type ω-transaminase of the present invention are as follows:
[0065] (1) Construction of the template:
[0066] Under the premise of not changing the amino acid sequence, the optimized transaminase gene taA (sequence As shown in SEQ ID NO.: 4, the corresponding amino acid sequence is shown in SEQ ID NO.: 5), the synthetic taA gene was connected to the vector pUC57 to obtain the recombinant plasmid pUC-taA, and then restricted by Nde Ⅰ and Xho Ⅰ Recombinant plasmid pUC-taA and vector pET-22b(+) were simultaneously digested with endonuclease, and the digested product was ligated with T4DNA ligase, and the ligated product was transformed into competent cells of Escherichia coli DH5α strain. After revived on a shaker, spread on LB petri dishes containing ampicillin with a final concentration of 50 μg / ml, and culture overnight in a 37°C incubator. Pick a single ...
Embodiment 2
[0082] Embodiment 2: Activity experiment 1 of R type ω-transaminase
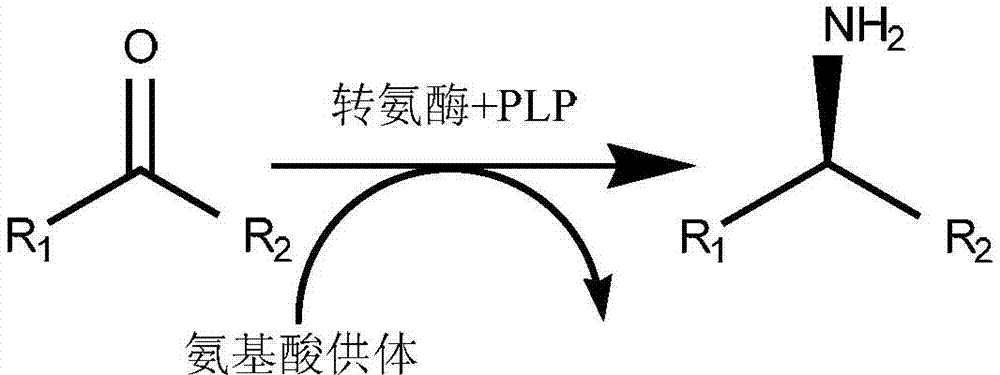
[0083] Add 1 g of the main raw material (N-BOC-piperidone, CAS: 79099-07-3) and 1 mL of dimethyl sulfoxide into the reaction flask. Triethanolamine buffer solution, 0.765g isopropylamine, 0.01g pyridoxal phosphate and 0.01g above-mentioned amino acid sequence as shown in SEQ ID NO.: 2 triethanolamine buffer solution, 0.765g isopropylamine, 0.01g above-mentioned amino acid sequence as shown in SEQ ID NO.: 2, the pH of the system is 9.5 , 30 ℃ constant temperature stirring for 12h. The pH of the system was adjusted to above 10 with 2N NaOH, extracted twice with ethyl acetate, the organic phase was dried, filtered, and concentrated to obtain the crude product (Chinese name: (R)-1-N-Boc-3-aminopiperidine, CAS: 188111-79-7), detected by gas chromatography (GC), the conversion rate was 90.8%, and the e.e value was 100%.
[0084] The nuclear magnetic data of gained product is as follows: 1H-NMR (300MHz, CDCl3) de...
Embodiment 3
[0085] Embodiment 3: Activity experiment 2 of R type ω-transaminase
[0086] Add 0.1g of the main raw material (2,4-dichloroacetophenone, CAS: 2234-16-4) and 1.5mL of polyethylene glycol PEG-400 into the reaction bottle, after the raw material is dispersed, add 23.5ml of phosphate buffer (pH8.0), 0.031g of isopropylamine, 0.0075g of pyridoxal phosphate and 0.02g of the mutant R-type ω-transaminase whose amino acid sequence is shown in SEQ ID NO.: 2, the pH of the system is 8.0, and the temperature is stirred at 45°C 20h. The pH of the system was adjusted to above 10 with 2N NaOH, extracted twice with ethyl acetate, the organic phase was dried, filtered, and concentrated to obtain the crude product ((R)-2,4-dichlorophenethylamine), detected by GC, with a conversion rate of 95 %, e.e. value 100%.
[0087] The nuclear magnetic data of gained product is as follows: 1H NMR (400MHz, DMSO D6): δ=7.67 (d 1H), 7.60 (d, 1H), 7.47 (dd, 1H), 7.34 (dd, 4H), 7.23-7.12 (m , 6H), 4.84 (s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com