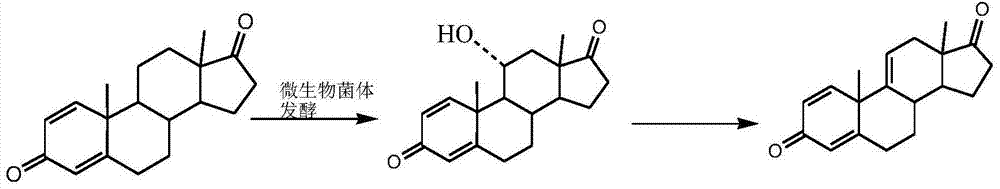
Preparation method of 1,4,9(11)-triene-androst-3,17-dione
A technology of triene androster and dione, which is applied in the field of preparation of 1,4,9(11)-triene androst-3,17-dione, can solve the problem of high production cost and the price of starting materials High, low production yield and other problems, to achieve the effect of avoiding ultra-low temperature reaction, less side reactions, and less equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
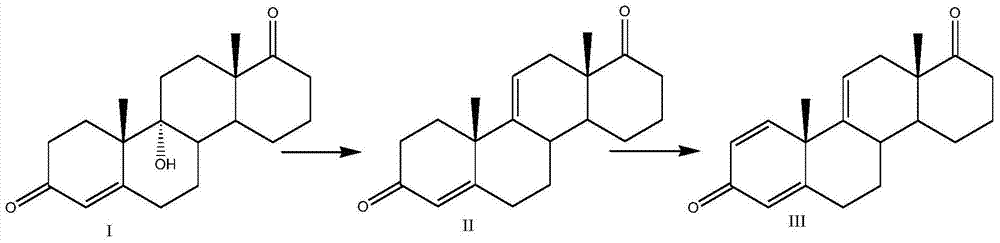
[0027] Put 500ml of chloroform, 100g of 9ɑ-OH-4AD (compound I), and 10g of ferric chloride into the three-necked reaction flask, stir and heat up to reflux, keep the temperature for 5-6h, after the reaction is complete, cool down to about 25°C, add water dropwise 200ml to terminate the reaction, concentrated under reduced pressure to gradually dryness, added 1000ml of water, stirred for 30min, filtered and washed with water until neutral, dried to obtain 90g of compound 4,9(11)-diene androst-3,17-dione (compound II) ; Weight yield: 90%, HPLC content: 99%.
[0028] According to the formula: yeast extract 4g / L, peptone 4g / L, magnesium sulfate heptahydrate 0.5g / L, disodium hydrogen phosphate 4g / L, potassium dihydrogen phosphate 2g / L, glucose 10g / L, agar powder 20g / L, Prepare the slant seed medium, fully dissolve various raw materials according to the ratio during preparation, stir evenly, use 20% NaOH solution to adjust the pH value to 7.2, then raise the temperature to 121°C-122...
Embodiment 2
[0036] Put 700ml of dichloromethane, 100g of 9ɑ-OH-4AD (compound I), and 5g of antimony pentachloride into the three-necked reaction flask, stir and heat up to reflux, keep warm for 15-16h, after the reaction is complete, cool down to about 10°C, add water dropwise 200ml to terminate the reaction, concentrated under reduced pressure to gradually dryness, added 1000ml of water, stirred for 30min, filtered and washed with water until neutral, dried to obtain 89g of compound 4,9(11)-diene androst-3,17-dione (compound II) ; Weight yield: 89%, HPLC content: 99%.
[0037] According to the formula: yeast extract 4g / L, peptone 4g / L, magnesium sulfate heptahydrate 0.5g / L, disodium hydrogen phosphate 4g / L, potassium dihydrogen phosphate 2g / L, glucose 10g / L, agar powder 20g / L, Prepare the slant seed medium, fully dissolve various raw materials according to the ratio during preparation, stir evenly, use 20% NaOH solution to adjust the pH value to 7.2, then raise the temperature to 121°C-1...
Embodiment 3
[0045] Put 100ml of toluene, 100ml of glacial acetic acid, 100g of 9ɑ-OH-4AD (compound I) into the three-necked reaction flask, cool down to about 10°C, add 50ml of 70wt% sulfuric acid dropwise, after the dropwise addition, react at a constant temperature of 10°C for 20h, and add 400ml of water dropwise Terminate the reaction, concentrate toluene under reduced pressure, add 1000ml of water, stir for 30min, filter and wash with water until neutral, and dry to obtain 87g of compound 4,9(11)-diene androst-3,17-dione (compound II); weight Yield: 87%, HPLC content: 97%.
[0046] According to the formula: yeast extract 4g / L, peptone 4g / L, magnesium sulfate heptahydrate 0.5g / L, disodium hydrogen phosphate 4g / L, potassium dihydrogen phosphate 2g / L, glucose 10g / L, agar powder 20g / L, Prepare the slant seed medium, fully dissolve various raw materials according to the ratio during preparation, stir evenly, use 20% NaOH solution to adjust the pH value to 7.2, then raise the temperature to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com