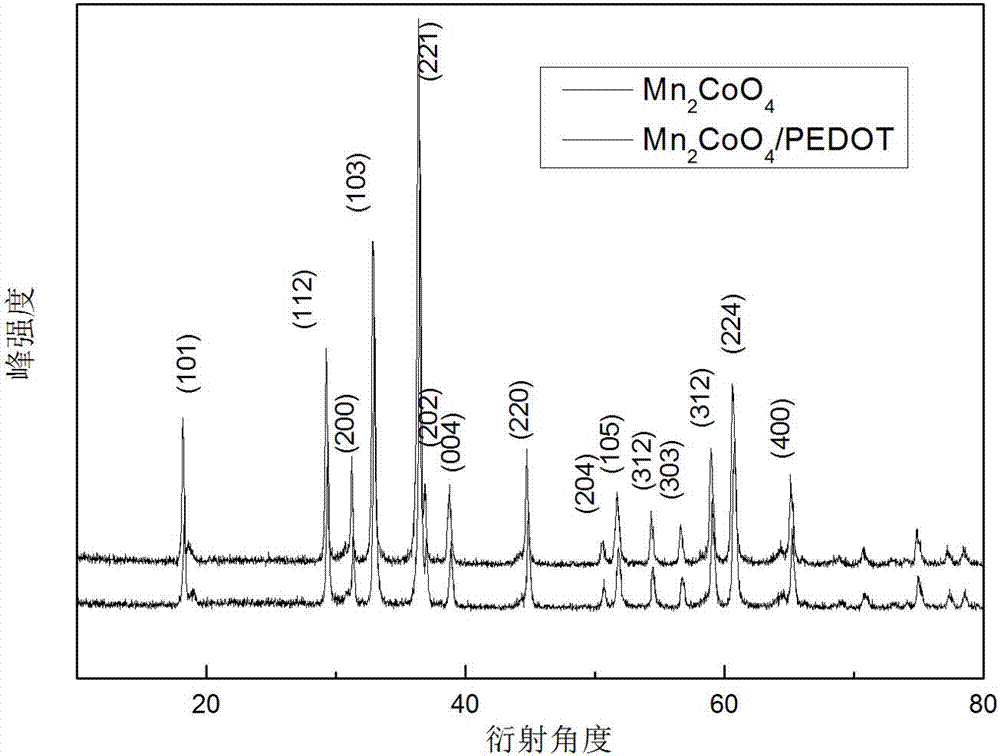
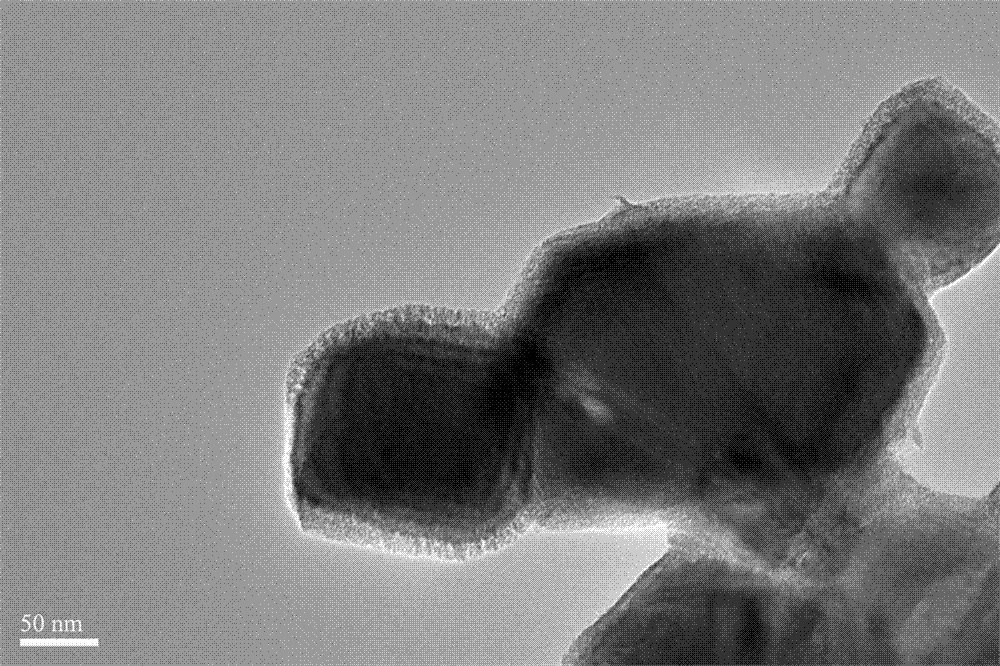
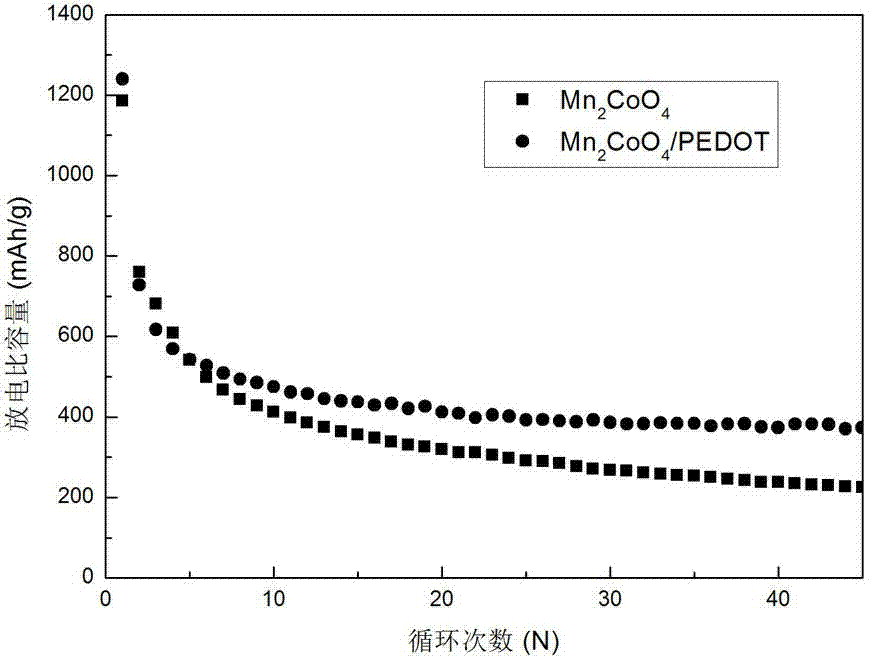
Preparation method of negative electrode material Mn2CoO4/poly(3,4-ethylenedioxythiophene)
A technology of ethylenedioxythiophene and negative electrode materials, which is applied in the direction of battery electrodes, secondary batteries, electrical components, etc., can solve the problems that batteries cannot realize high-current charging and discharging, limit the application range of lithium-ion batteries, and short-circuit lithium dendrites.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Dissolve manganese acetate and cobalt acetate in deionized water at a molar volume of 0.01 mol: 0.005 mol; (2) Add a small amount of 0.015 -0.030 mol ethylenediaminetetraacetic acid (EDTA)-polyacrylic acid (PPA) double chelating agent Wet with deionized water, add 0.15 mol (12 ml) ammonia water, shake until a colorless and transparent solution is formed, then add the colorless and transparent double chelating agent EDTA-PPA solution into the saline solution to form a transparent solution, heat and stir at 80 ℃ until the formation gel. (3) Heat and dry the gel material in a blast oven at 240°C for 5 hours; (4) Grind the precursor and calcinate it in a muffle furnace at 600°C for 10 hours at a heating rate of 5°C / min to obtain Mn 2 CoO 4 . (5) Dissolve 0.4 g of iron p-toluenesulfonate hexahydrate in 1 ml of absolute ethanol to a yellow transparent solution that is uniformly stirred, and then add 0.5 g of Mn 2 CoO 4 Add to this solution and stir until evenly spread...
Embodiment 2
[0029] (1) Dissolve manganese acetate and cobalt acetate in deionized water at a molar volume of 0.01 mol: 0.005 mol; (2) Add a small amount of 0.015-0.030 mol ethylenediaminetetraacetic acid (EDTA)-citric acid (CA) double chelating agent Wet with deionized water, add 0.15 mol (12 ml) of ammonia water, shake until a colorless and transparent solution is formed, then add the colorless and transparent double chelating agent EDTA-CA solution into the saline solution to form a transparent solution, heat and stir at 70°C until it forms gel. (3) Heat and dry the gel material in a blast oven at 240°C for 5 hours; (4) Grind the precursor and calcinate it in a muffle furnace at 800°C for 5 hours at a heating rate of 2°C / min to obtain Mn 2 CoO 4 . (5) Dissolve 0.4 g of iron p-toluenesulfonate hexahydrate in 1 ml of acetone until a uniformly stirred yellow transparent solution is obtained, and then add 0.5 g of Mn 2 CoO 4 Add to this solution and stir until evenly spread on a clean g...
Embodiment 3
[0031](1) Dissolve manganese acetate and cobalt acetate in deionized water at a molar volume of 0.01 mol: 0.005 mol; (2) Add a small amount of deionized water to 0.015 -0.030 mol of ethylenediaminetetraacetic acid (EDTA)-acetylacetone double chelating agent Wet, add 0.15 mol (12 ml) ammonia water, shake until a colorless and transparent solution is formed, then add the colorless and transparent double chelating agent EDTA-acetylacetone solution into the saline solution to form a transparent solution, heat and stir at 80°C until a gel is formed . (3) Heat and dry the gel material in a blast oven at 200 °C for 5 hours; (4) Grind the precursor and calcinate it in a muffle furnace at 850 °C for 5 hours at a heating rate of 2 °C / min to obtain Mn 2 CoO 4 . (5) Dissolve 0.2 g of iron p-toluenesulfonate hexahydrate in 0.5 ml of acetone until a uniformly stirred yellow transparent solution is obtained, and then add 0.25 g of Mn 2 CoO 4 Add to this solution and stir until evenly spr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com