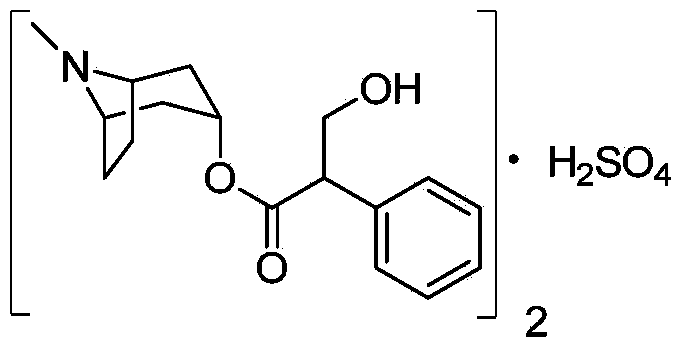
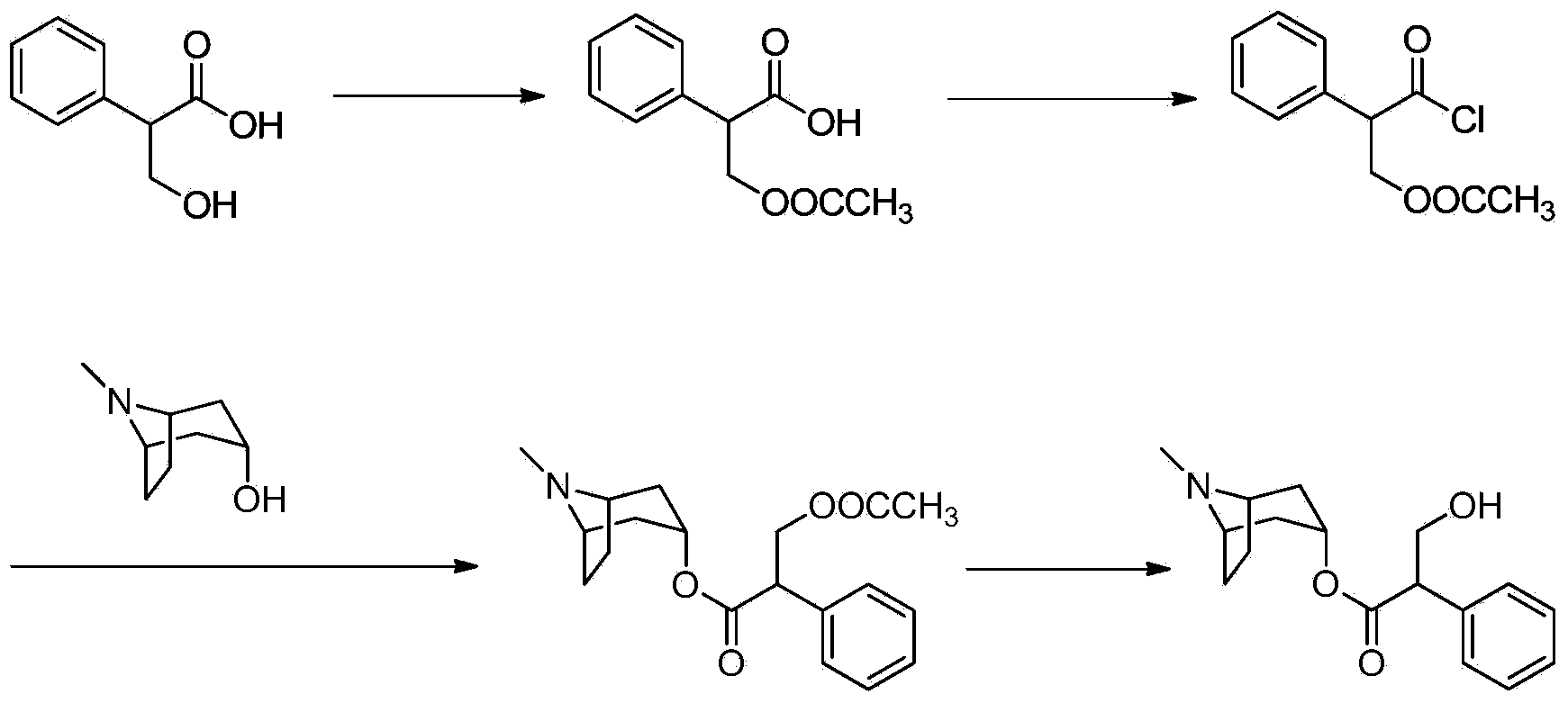
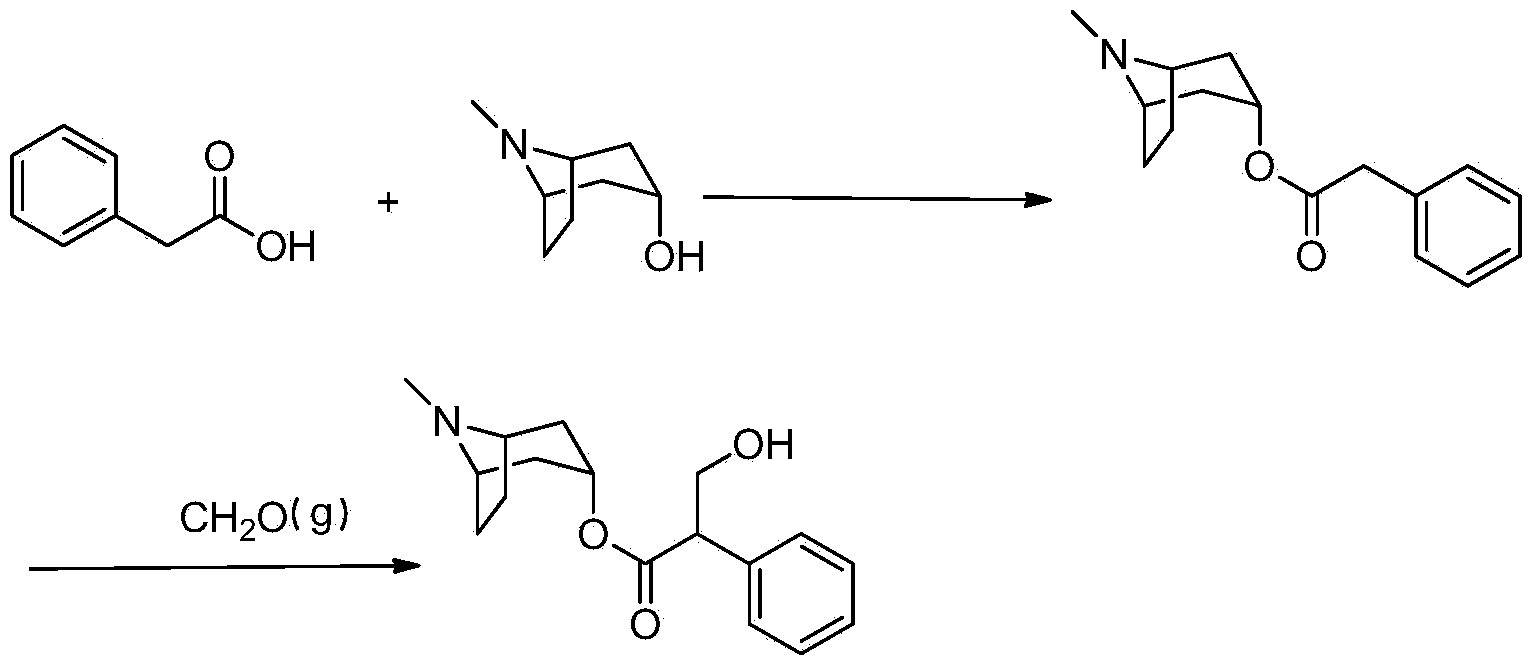
Preparation method of anticholinergic agent atropine sulphate
A technology of atropine sulfate and atropine, applied in the direction of organic chemistry, can solve the problems of high requirements for reaction equipment, unfavorable industrial production, harsh reaction conditions, etc., and achieve the effect of low production cost, low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation method of compound III
[0032] Add 250g (1.66mol) of methyl phenylacetate (compound II), 66.75g (1.66mol) of sodium hydroxide into a 1L three-necked flask, and then add 833ml of purified water and stir to raise the temperature to 70-85°C. After reacting for 4-5 hours, the temperature was lowered to about 30°C, and concentrated hydrochloric acid was added to adjust the pH to 1, and a large amount of white solid was precipitated immediately. Stir and crystallize, filter and dry with suction to obtain 185 g of compound III as a white solid with a yield of 85%.
Embodiment 2
[0033] Embodiment 2: the preparation method of compound IV
[0034] Add 100g (0.73mol) of phenylacetic acid (compound III) and 105g (0.88mol) of thionyl chloride into a 1L three-necked flask, then add 500ml of dichloromethane, add DMF as a catalyst, stir and heat up to 40°C under reflux, and react for 5 to 6 hours Finally, the solution was transferred to a 500ml single-necked flask and spin-dried. 110 g of light yellow liquid was obtained. Yield 97%
Embodiment 3
[0035] Embodiment 3: the preparation method of compound V
[0036] Add 110g (0.71mol) of compound IV to a 500ml three-necked flask and stir at room temperature, add 93.3g (0.66mol) of tropine alcohol and 80ml of chloroform into a 500ml beaker and stir until dissolved, and slowly drop the prepared tropine alcohol solution Add it to a three-necked flask, heat up vigorously, and react at 70-85°C for 6-7h. After the reaction is over, pour the reaction solution into a 2L beaker, stir, add 800-1000L of purified water, a large amount of white solids are precipitated, adjust the pH to 1-2 with 5% HCl solution, let stand for stratification, and separate the acidic water layer, three Keep the chloromethane layer; add 5% HCl to the chloroform layer to readjust the pH to 1~2, and let it stand for stratification; separate the water layer, combine the above water layers, wash twice with 50*2 dichloromethane, and let stand Separate the layers, separate the water layer, discard the dichloro ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com