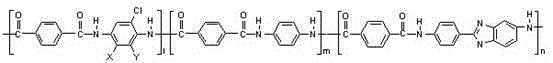High-performance chloric heterocyclic aramid fiber as well as preparation method and application
A heterocyclic aramid, high-performance technology, applied in the field of special synthetic fibers, can solve problems such as not having too many advantages, and achieve the effect of improving surface properties, good wettability and binding force, and improving surface activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
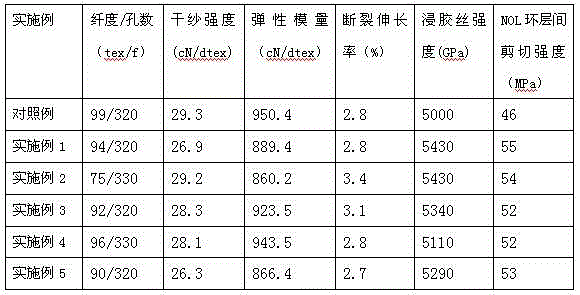
Examples
Embodiment 1
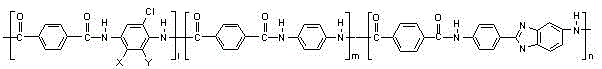
[0036] A. Combine 2,5-dichloro-p-phenylenediamine (15.35g), p-phenylenediamine (18.73g), 2-(4-aminophenyl)-5-aminobenzene with a molar ratio of 0.34:0.66:1 Add imidazole (58.27g) to 4000g of dimethylacetamide solution containing 3.5% lithium chloride (140g) by mass, stir at 25℃ for 1 hour under nitrogen protection, then cool to 7℃, then Add 105.62 grams of terephthaloyl chloride in 3 batches, stir and react for 2 hours to obtain a polymer solution with a polymer solid content of 4.0% and a dynamic viscosity of 85,000 cps;
[0037] B. The above polymer solution is degassed at normal pressure, filtered and then subjected to wet spinning. The polymer solution is sprayed into dimethylacetamide with a mass fraction of 50% dimethylacetamide at 20°C through a 320*0.09mm spinneret. The nascent fiber is formed in the acetamide aqueous solution, and the nascent fiber is stretched by 110% in a dimethylacetamide aqueous solution with a mass fraction of 20% dimethylacetamide at 60°C, and wash...
Embodiment 2
[0039] A. Combine 2,5-dichloro-p-phenylenediamine (2.83g), p-phenylenediamine (15.59g) and 2-(4-aminophenyl)-5-aminobenzene with a molar ratio of 0.05:0.45:1 Add imidazole (71.84g) to 3820g of dimethylacetamide solution containing 3.5% lithium chloride (133.7g) by mass, stir at 35℃ for 40 minutes under nitrogen protection, and then cool to 5℃. Then a total of 97.65 g of terephthaloyl chloride was added in 2 batches, and after stirring and reacting for 2 hours, a polymer solution with a polymer solid content of 4.0% and a dynamic viscosity of 100,000 cps was obtained;
[0040] B. Degas the above polymer solution at normal pressure, filter, and perform wet spinning. The polymer solution is sprayed into dimethylacetamide with a mass fraction of 50% dimethylacetamide at 20°C through a 330*0.08mm spinneret. The nascent fiber is formed in the acetamide aqueous solution, and the nascent fiber is stretched by 110% in a dimethylacetamide aqueous solution with a mass fraction of 20% dimeth...
Embodiment 3
[0042] A. Combine 2-chloro-p-phenylenediamine (7.99 g), p-phenylenediamine (24.24 g), 2-(4-aminophenyl)-5-aminobenzimidazole ( 62.84g) was added to 4000g of dimethylacetamide solution containing 3.0% lithium chloride (120g) by mass, stirred at 30℃ for 1 hour under nitrogen protection, cooled to 7℃, and then divided into 3 batches A total of 113.89 grams of terephthaloyl chloride was added, and after stirring and reacting for 2.5 hours, a polymer solution with a polymer solid content of 4.2% and a dynamic viscosity of 110,000 cps was obtained;
[0043] B. The above polymer solution is degassed at normal pressure, filtered and then subjected to wet spinning. The polymer solution is sprayed into dimethylacetamide with a mass fraction of 50% dimethylacetamide at 20°C through a 320*0.09mm spinneret. The nascent fiber is formed in the acetamide aqueous solution, and the nascent fiber is stretched by 110% in a dimethylacetamide aqueous solution with a mass fraction of 20% dimethylacetam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dynamic viscosity | aaaaa | aaaaa |
| Dynamic viscosity | aaaaa | aaaaa |
| Dynamic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com