A preparation method of androsta-17β-n-(2,5-bis(trifluoromethyl))benzamide
A technology of trifluoromethyl and benzamide, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of weak nucleophilic attack ability, large amount of pyridine used, slow reaction and the like, achieves enhanced nucleophilic attack ability, facilitates industrialized implementation, Yield-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 117
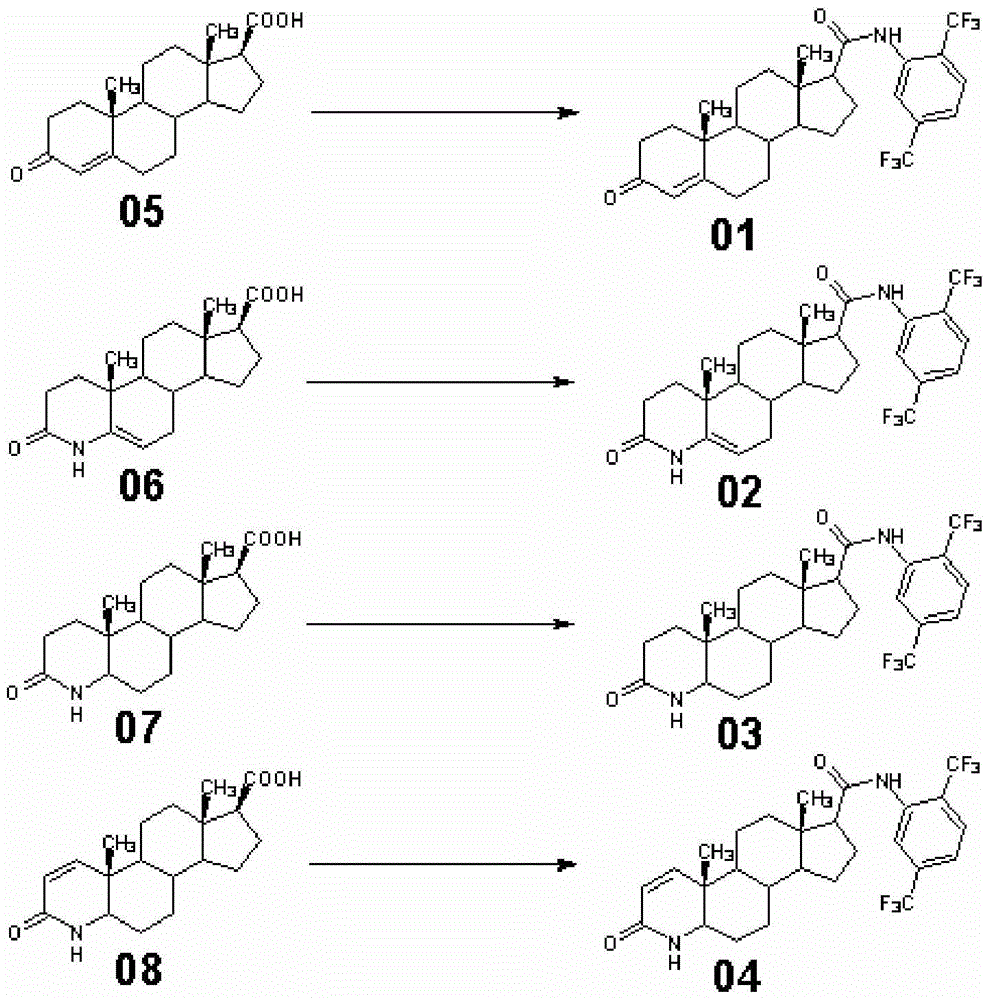
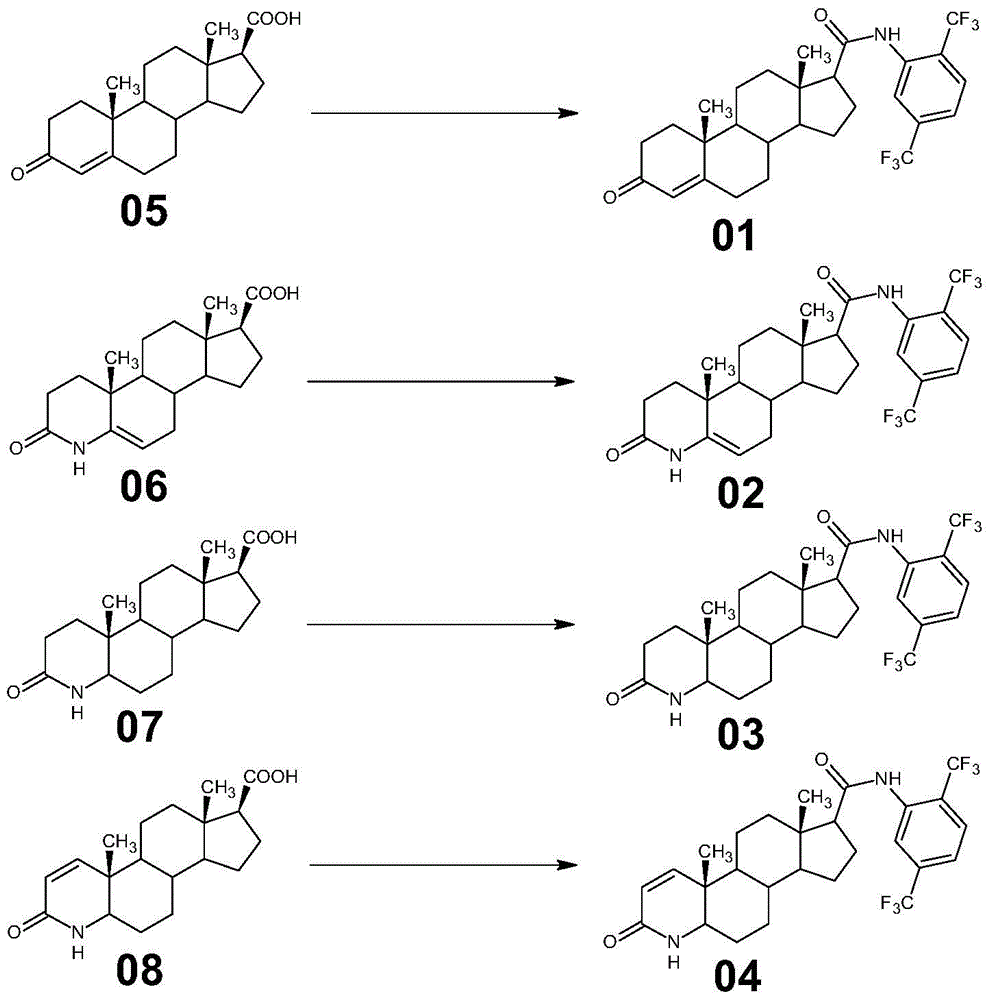
[0039] Example 1 Preparation of 17β-N-(2,5-bis(trifluoromethyl))benzamido-4-androsten-3-one
[0040] A. At room temperature, in a 2000L enamel amide reaction kettle equipped with a thermometer and a stirrer, pump 300kg of toluene and 85kg of 2,5-bis(trifluoromethyl)aniline, stir, circulate water through the jacket, and slowly add them dropwise 1M methylmagnesium chloride tetrahydrofuran solution 360kg, stirred and kept warm for later use;
[0041] B. At room temperature, in a 2000L enamel amide reaction kettle equipped with a thermometer and agitator, pump 700kg of toluene, stir, add 4DMAP 5kg, fine powder anhydrous potassium carbonate 260kg, 3-keto-4-androstene-17β carboxylate Acid (05) 100kg, cooling water was passed through the jacket, 45kg of thionyl chloride was added dropwise at 20°C to 30°C, and the plate was spotted for 3 hours after the dropwise addition until the basic reaction was complete, and water was added for quick washing three times (1000kg of water was added...
Embodiment 217
[0042] Example 2 Preparation of 17β-N-(2,5-bis(trifluoromethyl))benzamido-4aza-5-androsten-3-one
[0043]The preparation method is the same as in Example 1, the only difference is that in this example, the substrate 3-keto-4-androstene-17β carboxylic acid (05) is replaced by 3-keto-4aza-5-androstene-17β Carboxylic acid (06). Finally, 148.95kg of 17β-N-(2,5-bis(trifluoromethyl))benzamido-4aza-5-androsten-3-one (02) was obtained, the weight yield was 148.95%, and the molar yield The rate is 89.43%.
Embodiment 317
[0044] Example 3 Preparation of 17β-N-(2,5-bis(trifluoromethyl))benzamido-4azandrost-3-one
[0045] The preparation method is the same as in Example 1, the only difference being that in this example, the substrate 3-keto-4-androstene-17β carboxylic acid (05) is replaced with 3-keto-4 azaandrostene-17β carboxylic acid ( 07). Finally, 153.86kg of 17β-N-(2,5-bis(trifluoromethyl))benzamido-4azandrost-3-one (03) was obtained, with a weight yield of 153.86% and a molar yield of 92.61%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com