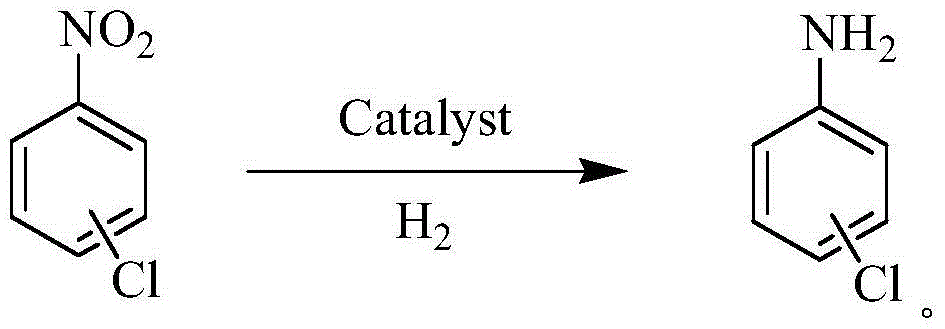
Method and catalyst for synthesizing chloroaniline from chloronitrobenzene by virtue of selective catalytic hydrogenation
A technology for chlorinated nitrobenzene and chlorinated aniline, applied in the field of synthesizing chlorinated aniline, can solve the problems of reduced reaction yield, easy dechlorination, catalyst poisoning and the like, and achieves high catalytic activity, good stability and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Preparation of selective hydrogenation supported bimetallic catalyst
[0024] Crush activated carbon, sieve activated carbon within the range of 200-300 mesh, add activated carbon and 17% HNO to a three-necked flask 3 solution (solid-to-liquid mass ratio: 1:10), stirred and refluxed at 80°C for 3 hours, washed with distilled water until neutral, filtered, and dried at 110°C to obtain an activated carbon carrier. Add 4g of carrier and 60ml of distilled water in a 250ml three-necked flask, and add 3.3ml of chloroplatinic acid solution (12.15g / L) and 0.81g of CoCl under stirring 2 .6H 2 O, heated to 80 °C, stirred for 2h; then added Na 2 CO 3 solution, adjust the pH to 10, stir for 30 min, and wash with deionized water several times until no Cl ions exist in the filtrate. Then drop to room temperature, add 2.1g NaBH under stirring condition 4 Carry out reduction for 2h, wash with deionized water several times until the filtrate is neutral, and dry at room...
Embodiment 2
[0028] Embodiment 2 The impact of different supported bimetallic catalysts on the selective hydrogenation of 4-chloronitrobenzene
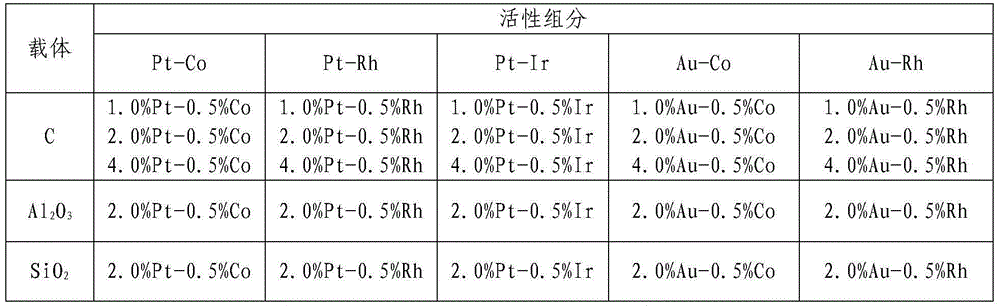
[0029] Weigh 15 mg of the catalyst prepared in Example 1, add it to a 100 ml three-necked flask, add 80 ml of methanol solution of 4-chloronitrobenzene with a concentration of 50 mmol / L, and stir the reactant with a magnetic stirrer for 5 min; then Pass N 2 , do this three times, and then pass H 2 , H 2 The flow rate is controlled within the range of 10-15ml / min, the reaction temperature is controlled at 30°C, and the reaction pressure is normal pressure. Depending on the selective hydrogenation rate of 4-chloronitrobenzene catalyzed by different catalysts, the reaction time is also different. Same, the specific dechlorination results are shown in Table 2.
[0030] Table 2 Effects of different supported bimetallic catalysts on the selective hydrogenation of 4-chloronitrobenzene
[0031] Reaction substrate
Embodiment 32
[0032] The effect of embodiment 32.0%Pt-0.5%Rh / C on the selective hydrogenation of different chloronitrobenzenes
[0033] Take by weighing the 2.0%Pt-0.5%Rh / C catalyst that 15mg embodiment 1 prepares, join in the there-necked flask of 100ml, add concentration and be the methanol solution 80ml of the chlorinated nitrobenzene of 50mmol / L, stir under magnetic stirrer Allow the reactant to be adsorbed for 5min; then pass N 2 , do this three times, and then pass H 2 , H 2 The flow rate is controlled within the range of 10-15ml / min, the reaction temperature is controlled at 30°C, and the reaction pressure is normal pressure. Depending on the selective hydrogenation rate of different reaction substrates, the reaction time is also different. The specific dechlorination results See Table 3.
[0034] Table 32.0%Pt-0.5%Rh / C Effect on Selective Hydrogenation of Different Chloronitrobenzenes
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com