Method for preparation of aryl amides by aromatic carboxylic acid amination
A technology of aryl carboxylic acid amine and aryl amide, which is applied in the preparation of carboxylic acid amide, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of low activity, difficult-to-separate by-products, poor product selectivity, etc., Achieve high activity, reduce toxicity and cost, and the product does not contain salt impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
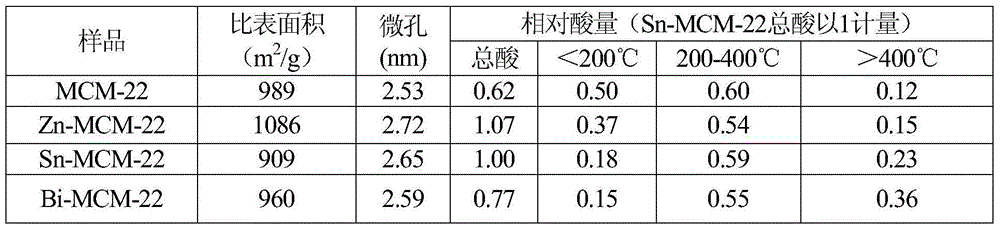
[0023] Using silica sol as the silicon source, aluminum nitrate as the aluminum source, hexamethyleneimine (HMI) as the template, adding sodium hydroxide and water to adjust the sol, NaOH, Al(NO 3 ) 2 ·9H 2 O, SiO 2 , H 2 The ratio of the amount of O and HMI is 20:3:100:4000:6, magnetically stirred, and crystallized at 60°C for 7 days. The synthesized product is filtered, washed, dried and calcined at 600°C to remove the template. Then ion exchange is carried out with zinc nitrate with a mass concentration of 2%, and Zn-MCM-22 is obtained after roasting.
[0024] Adopt Micromeritics TriStar II3020 adsorption instrument and NH 3 The specific surface area, pore size and acid distribution of the sample Zn-MCM-22 were measured by temperature programmed desorption. The sample test data is shown in Table 1.
Embodiment 2
[0026] Using silica sol as the silicon source, aluminum nitrate as the aluminum source, hexamethyleneimine (HMI) as the template, adding sodium hydroxide and water to adjust the sol, NaOH, Al(NO 3 ) 2 ·9H 2 O, SiO 2 , H 2 The ratio of the amount of O and HMI is 20:2:100:4000:5, magnetically stirred, and crystallized at 60°C for 7 days. The synthesized product is filtered, washed, dried and calcined at 600°C to remove the template. Then, ion exchange is carried out with tin nitrate solution with a mass concentration of 2%, and Sn-MCM-22 is obtained after roasting.
[0027] Adopt Micromeritics TriStar II3020 adsorption instrument and NH 3 The specific surface area, pore size and acid distribution of sample Sn-MCM-22 were measured by temperature programmed desorption. The sample test data is shown in Table 1.
Embodiment 3
[0029] Using silica sol as the silicon source, aluminum nitrate as the aluminum source, hexamethyleneimine (HMI) as the template, adding sodium hydroxide and water to adjust the sol, NaOH, Al(NO 3 ) 2 ·9H 2 O, SiO 2 , H 2 The ratio of the amount of O and HMI is 20:1:100:4000:5, magnetic stirring, crystallization at 60°C for 7 days, the synthesized product is filtered, washed, dried and calcined at 600°C to remove the template, then The bismuth nitrate solution with a mass concentration of 2% is used for ion exchange, and Bi-MCM-22 is obtained after roasting.
[0030] Adopt Micromeritics TriStar II3020 adsorption instrument and NH 3 The specific surface area, pore size and acid distribution of Bi-MCM-22 were measured by temperature-programmed desorption. The sample detection data are shown in Table 1.
[0031] Table 1 Comparison of physical and chemical properties of four synthetic molecular sieve samples
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com