Water-soluble magnetic resonance imaging contrast agent containing nitroimidazole group and preparation method of contrast agent
A nitroimidazole-based, magnetic resonance imaging technology, applied in preparations for in vivo experiments, pharmaceutical formulations, etc., can solve the problems of unsatisfactory organ imaging, shorten the longitudinal relaxation time, improve sensitivity and specificity, Effect of increasing paramagnetic resonance signal intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] EDTA-α,δ-bis[1-(2-nitro-1H-imidazole)]ethanol diester metal gadolinium(Ⅲ) chelate
[0054]
[0055] Step 1. Preparation of ethylenediaminetetraacetic acid cyclodianhydride (EDTAA)
[0056] Weigh 5.80g (0.02mol) of ethylenediaminetetraacetic acid (EDTA) into a 50mL round bottom flask, add 8mL (0.08mol) of acetic anhydride and 10mL (0.12mol) of pyridine, and install a straight condenser with a drying tube above the flask Stir and reflux at 65°C for 24 hours, cool to room temperature, filter with suction, wash with acetic anhydride, cold DMF and ether, recrystallize with DMF-ether, and dry in vacuo to obtain a white powder called ethylenediaminetetraacetic acid cyclobis Anhydride (EDTAA), 74% yield. m.p. is 189-191°C; elemental analysis measured value (%, calculated value): C 46.51 (46.87), H 4.98 (4.69), N 10.27 (10.94).
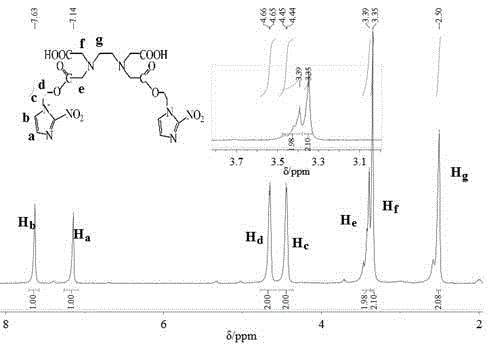
[0057] Step 2. EDTA α,δ-bis[1-(2-nitro-1H-imidazole)]ethanol diester (L 1 )Synthesis
[0058] Add 1.63g (6mmol) ethylenediaminetetraacetic acid ...
Embodiment 2
[0065] Diethylenetriaminepentaacetic acid-α,η-bis[1-(2-nitro-1H-imidazole)]ethanol diester metal gadolinium(Ⅲ) chelate
[0066]
[0067] Step 1. Preparation of diethylenetriaminepentaacetic acid cyclodianhydride (DTPAA)
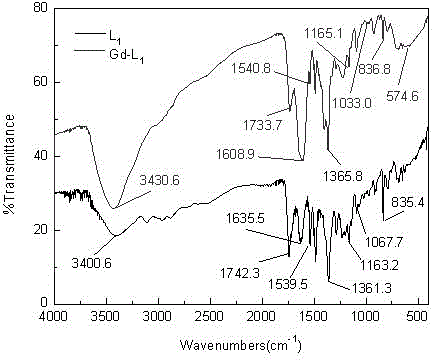
[0068] Weigh 7.86g (0.02mol) of diethylenetriaminepentaacetic acid into a 50mL three-neck flask, add 15.0mL of anhydrous pyridine, slowly add 8.0mL (0.08mol) of acetic anhydride dropwise under nitrogen protection, and stir and reflux at 60°C for 24h , cooled to room temperature, suction filtered, the resulting solid was washed with acetic anhydride until no amber, recrystallized from DMF-ether to obtain 6.68 g of white powder, yield 85%; m.p.189~191°C (literature value: 190°C); FT- IR (KBr, cm -1 ): 3430.7(n OH ), 1820.7 and 1773.5 (n 酸酐羰基 ); 1639.7(n COOH ),1108.4(n C-O-C ).Anal.calculated for C 14 h 19 N 3 o 8 : C 47.06, H 5.32, N 11.76; found C 46.85, H 5.52, N 11.58.
[0069] Step 2. Diethylenetriaminepentaacetic acid-α, η-bis[1-(2-nitro-1H-i...
Embodiment 3
[0077] EDTA-α,δ-bis[1-(2-methyl-5-nitro-1H-imidazole)]ethanol diester metal gadolinium(Ⅲ) chelate
[0078]
[0079] Step 1. Preparation of ethylenediaminetetraacetic acid cyclodianhydride (EDTAA)
[0080] Weigh 5.80g (0.02mol) of ethylenediaminetetraacetic acid (EDTA) into a 50mL round bottom flask, add 8mL (0.08mol) of acetic anhydride and 10mL (0.12mol) of pyridine, and install a straight condenser with a drying tube above the flask Stir and reflux at 65°C for 24h, cool to room temperature, filter with suction, wash with acetic anhydride, cold DMF and ether, recrystallize with DMF-ether, and dry in vacuo to obtain a white powder called ethylenediaminetetraacetic acid cyclobis Anhydride (EDTAA), 74% yield. m.p. is 189-191°C; elemental analysis measured value (%, calculated value): C 46.51 (46.87), H 4.98 (4.69), N 10.27 (10.94).
[0081] Step 2. EDTA-α,δ-bis[1-(2-methyl-5-nitro-1H-imidazole)]ethanol diester (L 3 )Synthesis
[0082] Add 1.20g (4.8mmol) ethylenediaminete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com