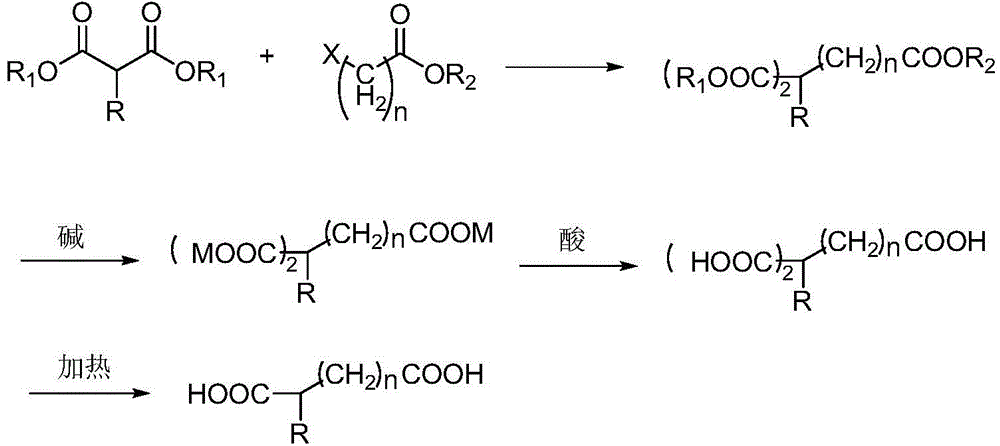
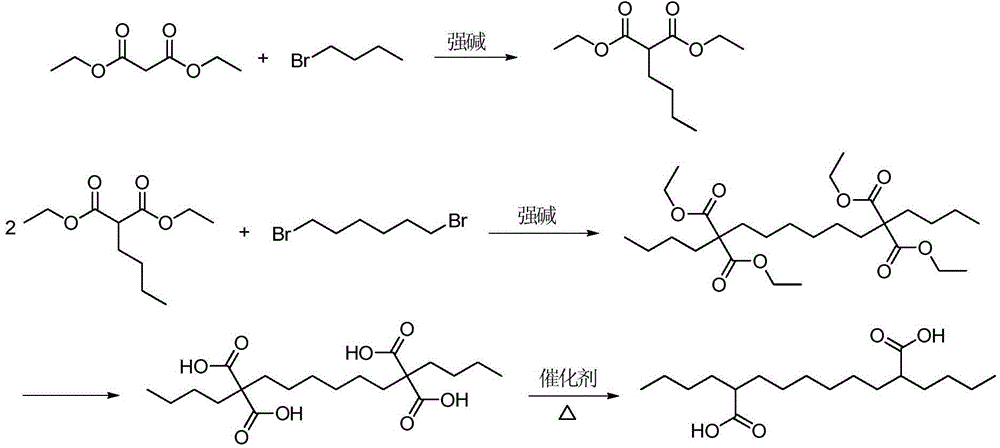
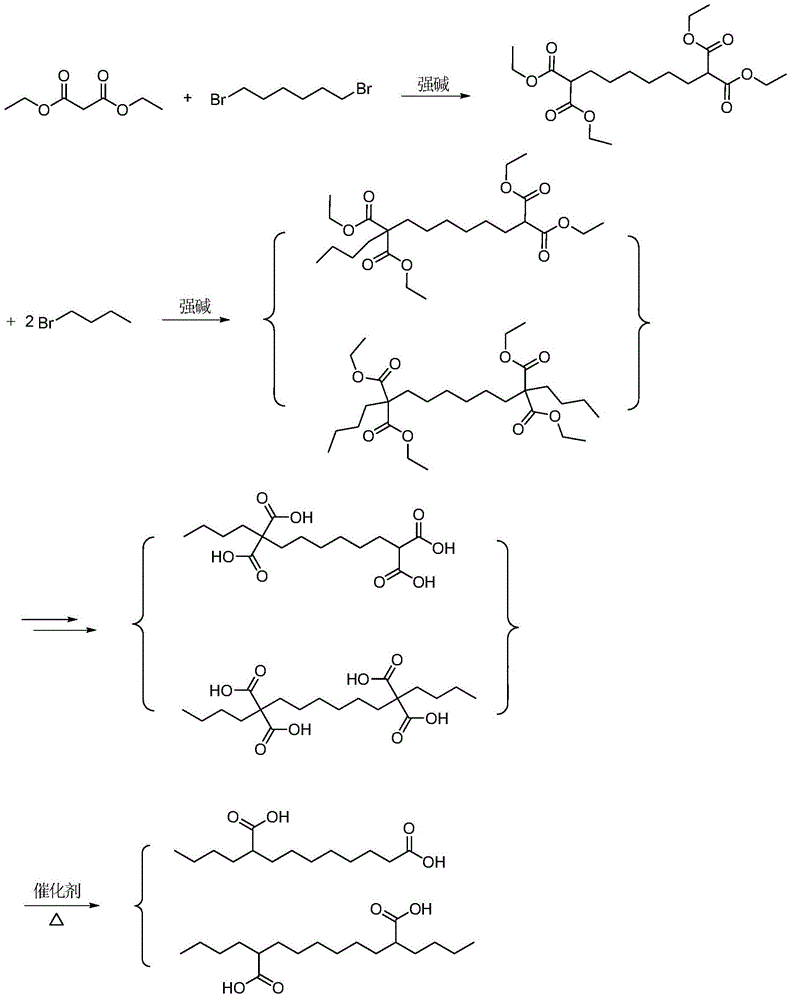
Co-production method for capacitance-grade 2-butylsebacic acid and 2, 9-butylsebacic acid
A technology of butyl sebacic acid and diethoxycarbonyl diethyl sebacate, applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation and other directions, can solve problems such as low oxidation efficiency of electrolyte , to achieve the effect of excellent electrochemical performance, improved oxidation efficiency and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1: Add 1922.4 g (12.0 mol) of diethyl malonate and 1463.8 g (6.0 mol) of 1,6-dibromohexane into a 10L three-necked flask, and dropwise add 4489.3 g of sodium ethoxide ethanol solution with a mass concentration of 20% g (of which 13.2 mol of sodium ethoxide), stirred under reflux for 8 hours after the dropwise addition, cooled to room temperature, and filtered to obtain ethyl 9,9-bis(methoxycarbonyl)-undecanecarboxylate (C) ethanol Solution 6803.3g.
[0029] Step 2: Carry out rectification of the above solution, collect the fraction with a top temperature of 165~200 ℃ (3mmHg), and obtain 2,9-bis(ethoxycarbonyl)-diethyl sebacate 2200.0g, step 1 and 2 The total yield was 91.1%, and the purity was 98.9% by gas phase detection.
[0030] Step 3: Add 2000g (4.97 mol) of 2,9-bis(ethoxycarbonyl)-diethyl sebacate and 1498.2g (10.9 mol) of n-bromobutane into a 10L three-necked flask, and the mass concentration of the dropwise addition is 3707.1g of 20% sodium ethoxide...
Embodiment 2
[0036] Step 1: Add 2403.0 g (15.0 mol) of diethyl malonate and 1463.7 g (6.0 mol) of 1,6-dibromohexane into a 10L three-necked flask, and dropwise add 4489.4 g of sodium ethoxide ethanol solution with a mass concentration of 20% g (13.2 mol), stirred under reflux for 8 hours after the dropwise addition, cooled to room temperature, and filtered to obtain 6860.1g ethanol solution of ethyl 9,9-bis(methoxycarbonyl)-undecanecarboxylate (C) .
[0037] Step 2: Distill the above solution, collect the fraction with a top temperature of 165~200°C (3mmHg), and obtain 2,9-bis(ethoxycarbonyl)-diethyl sebacate 2213.1g, step 1 and 2 The total yield was 91.6%, and the purity was 98.2% by gas phase detection.
[0038] Step 3: Add 2,9-bis(ethoxycarbonyl)-diethyl sebacate 2000g (4.97 mol) and n-bromobutane 1498.1g (10.9 mol) into a 10L three-necked flask. 20% sodium ethoxide ethanol solution 3707.2g (10.9 mol), after the dropwise addition was completed, stirred under reflux for 8 hours, c...
Embodiment 3
[0044] Step one, step two: with embodiment 1
[0045] Step 3: Add 2000g (4.97 mol) of 2,9-bis(ethoxycarbonyl)-diethyl sebacate and 1498.3g (10.9 mol) of n-bromobutane into a 20L three-necked flask, and the mass concentration of the dropwise addition is 7739.0g (10.9 mol) of 20% potassium carbonate ethanol solution, stirred under reflux for 8 hours after the dropwise addition, cooled to room temperature, and filtered to obtain 2-butyl-2,9-diethoxycarbonyl sebacic acid di 5483.5 g of ethyl ester and diethyl 2,9-dibutyl-2,9-diethoxycarbonyl sebacate in ethanol.
[0046] Step 4: Distill the above solution, collect the fraction with a top temperature of 170~180 ℃ (3mmHg), and obtain 454.0g of 2-butyl-2,9-diethoxycarbonyl sebacate (ester 1) , the total yield of the two-step ester 1 in steps 3 and 4 was 19.9%, and the fraction with a top temperature of 200~206°C (3mmHg) was collected to obtain 2,9-dibutyl-2,9-diethoxycarbonyldecanedi Acid diethyl ester (ester 2) was 1850.1g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com