Preparation and application of novel cysteine and homocysteine fluorescence probe
A technology of homocysteine and cysteine, which is used in the detection of sulfite ions, the preparation of new cysteine and homocysteine fluorescent probes, and can solve the problem of poor water solubility, etc. problem, to achieve the effect of low cost, good selectivity and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the synthesis of 2-compound 2-hydroxyl-6-acetylnaphthalene
[0019] Dissolve 2-methoxy-6-acetylnaphthalene (1.5170g, 7.58mmol) in 6mL of dichloromethane, then add 36% concentrated hydrochloric acid (100mL) in parts by mass, heat to 85°C and reflux for 4h, stop heating and cooling After reaching room temperature, the pH was adjusted to neutral with 50% sodium hydroxide solution, and a solid was precipitated. After filtration, the filter cake was obtained as the product, washed with distilled water three times, and dried in vacuum for 24 hours to obtain a white solid. Yield: 1.3552 g. Yield: 96%
Embodiment 2
[0020] Embodiment 2: the synthesis of probe molecule
[0021] 2-Hydroxy-6-acetylnaphthalene (0.0930g, 0.5mmol) was dissolved in 7mL of anhydrous dichloromethane, then triethylamine (0.1011g, 1mmol) was added, and 2,4-dinitrobenzenesulfonyl chloride (0.1333g, 0.5mmol) was dissolved in 3mL of anhydrous dichloromethane, slowly added dropwise to the reaction solution under an ice-water bath, continued to stir for 1h, stopped the reaction, and evaporated the solvent by column chromatography (eluent, V 二氯甲烷 / V 石油醚 =1 / 1) to obtain the product, dried in vacuo for 24h to obtain a pale yellow solid. Yield: 0.0882 g. Yield: 42.3%. The structure of the probe molecule is characterized as follows: 1 H NMR (400MHz, DMSO) δ9.15(d, J=2.3Hz, 1H), 8.74(s, 1H), 8.59(dd, J=8.7, 2.3Hz, 1H), 8.26(dd, J=14.2, 8.9Hz, 2H), 8.10-8.00(m, 2H), 7.90(d, J=2.4Hz, 1H), 7.43(dd, J=9.0, 2.5Hz, 1H), 2.71(s, 3H). 13 C NMR (126MHz, DMSO) δ198.17, 152.05, 148.63, 148.27, 135.79, 135.47, 134.12, 133.04, 131.70...
Embodiment 3
[0022] Embodiment 3: The present invention: the application of fluorescent probe
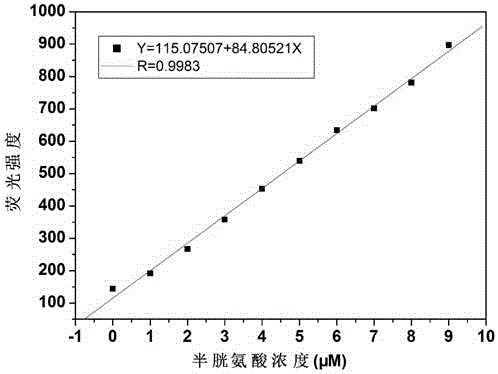
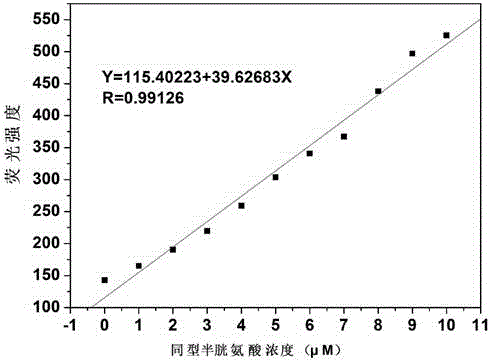
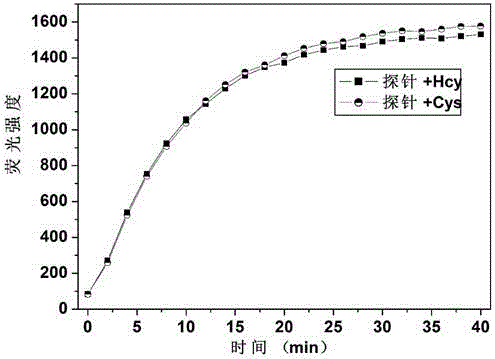
[0023] Dissolve the probe in the buffer solution (V 乙腈 / V HEPES =1 / 9, pH=7.4) to prepare 1.0×10 -5mol / L solution, adding amino acids (Asn, Ala, Val, Phe, His, Leu, Ser, Ile, Trp, Lys, Arg, Pro, Gly, Met, Tyr, Glu, Thr) to the solution did not cause fluorescence The addition of amino acids (Cys, Hcy) causes changes in fluorescence, and the fluorescent probe shows high sensitivity and high selectivity for cysteine and homocysteine. When cysteine and homocysteine coexist with interfering substances (Asn, Ala, Val, Phe, His, Leu, Ser, Ile, Trp, Lys, Arg, Pro, Gly, Met, Tyr, Glu, Thr) When , the probe is not affected by interference factors and shows a good anti-interference ability. The probe molecule reacts quickly with sulfite, and the change of fluorescence can be observed within 30 seconds. The probe molecule can selectively recognize sulfite in the pH range of 3 to 10, showing a wide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com