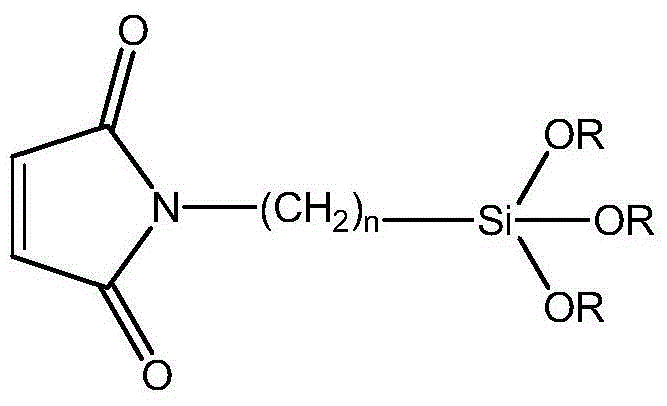
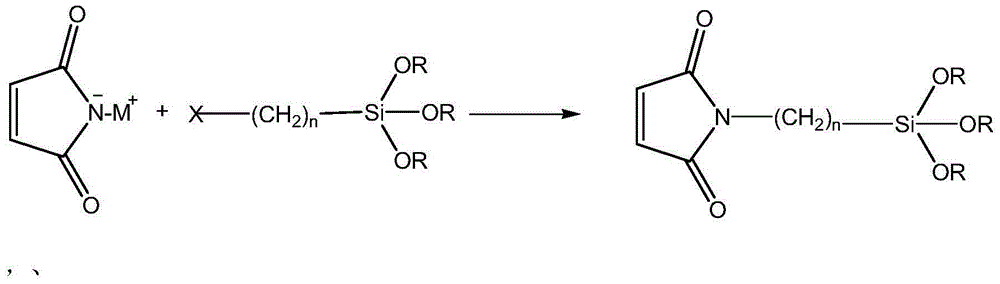
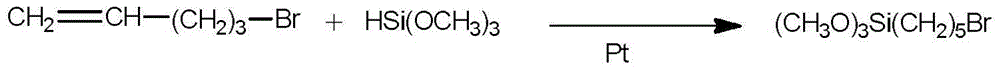Synthesis method of siloxane-substituted maleimide
A technology of maleimide and alkyl siloxane, which is applied in the field of polymer monomer synthesis, can solve the problems of easy hydrolysis of siloxane and cumbersome synthesis process, etc. convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 11-bromopentyltrimethoxysilane
[0029]
[0030] In an argon atmosphere, add trimethoxysilane (24.00g, 0.20mol) and bromopentene-1 (BrCH 2 CH 2 CH 2 CH=CH 2 ) (30.00g, 0.20mol) of the mixed solution, drop 30 drops of Karstedt catalyst (isopropanol solution of chloroplatinic acid). The mixture was heated to 80°C and maintained at this temperature for 24 hours. The obtained reaction mixture was quickly distilled under reduced pressure (below 100° C.) to obtain the target product with a yield of 30%. 1 H NMR (400MHz, CDCl 3 ,293K)δ:3.56(s,9H,OCH 3 ),3.37-3.42(s,2H,-CH 2 -Br),1.82-1.86(s,2H,-CH 2 -CH 2 -Br),1.38-1.45(s,4H,Si-CH 2 CH 2 CH 2 ),0.62-0.64(s,2H,Si-CH 2 ).
Embodiment 21
[0031] The synthesis of embodiment 21-bromohexyl triethoxysilane
[0032]
[0033] In an argon atmosphere, add trimethoxysilane (22.00 g, 0.18 mol) and bromohexene-1 (BrCH 2 CH 2 CH 2 CH 2 CH=CH 2 ) (30.00g, 0.18mol) of the mixed solution, drop 30 drops of Karstedt catalyst (isopropanol solution of chloroplatinic acid). The mixture was heated to 80°C and maintained at this temperature for 24 hours. The obtained reaction mixture was quickly distilled under reduced pressure (less than 100° C.) to obtain the target product with a yield of 35%. 1 H NMR (400MHz, CDCl 3 ,293K)δ:3.56(s,9H,OCH 3 ),3.37-3.42(s,2H,-CH 2 -Br),1.82-1.86(s,2H,-CH 2 -CH 2 -Br),1.38-1.45(s,6H,Si-CH 2 CH 2 CH 2 CH 2 ),0.62-0.64(s,2H,Si-CH 2 ).
Embodiment 31
[0034] Synthesis of Example 31-Bromododecyltrimethoxysilane
[0035] The synthesis of A, bromododecene-1
[0036]
[0037] Under cooling in an ice bath, bromohexene-1 (Br(CH 2 ) 4 CH=CH 2 ) (40.00g, 0.31mol), the addition was completed, and the mixture was stirred for 30 minutes under ice-bath cooling. The obtained Grignard reagent was transferred to the dropping funnel and slowly dropped into an ice-bath cooled mixture consisting of: Br(CH 2 ) 6 Br (77.4g, 0.4mol), Li 2 CuCl 4(0.1M solution in THF, 20ml) and THF (100ml). After dropping, the reaction mixture was further stirred for 30 minutes under cooling in an ice bath, and then the solvent was removed by evaporation. The obtained residue was treated with dilute hydrochloric acid and then extracted with n-hexane. After removal of the solvent, the crude product was obtained as an oil. Rectification under reduced pressure gave the pure product with a yield of 60%. 1 H NMR (400MHz, CDCl 3 ,293K)δ:5.75-5.85(s,1H,C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com