1-ferrocenyl-3-aryl-3-(1-acetyl-1-formylcarbethoxyl-methenyl)-acetone and preparation method thereof
A technology based on ethyl formate and ferrocene, which is applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve the problems of large solvent usage, low yield, long reaction time, etc. Short, simple reaction process, low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Weigh 0.0012mol ethyl acetoacetate, 0.0012mol anhydrous K 2 CO 3 Put it in a mortar and mix quickly and evenly, then add 0.001mol 1-ferrocenyl-3-phenyl-propenone, mix and grind. The mixture will become viscous as the reaction proceeds. Continue to grind until the substance does not change. Use thin-layer chromatography to monitor the reaction progress. After the reaction is completed, wash with pure water several times and filter to obtain a dark red solid, which is 1-2 Ferrocenyl-3-phenyl-3-(1-acetyl-1-carboxyethyl-methine)-propanone. Yield 80%, m.p. 125°C-126°C.
[0058] IR (KBr tablet, v / cm -1 ):2985, 1719, 1647, 1594, 1451, 1374, 1238;
[0059] 1 H-NMR: 7.20-7.71 (m, 5H, Ar-H), 4.97 (s, 2H, C 5 h 4 ), 4.65(s, 2H, C 5 h 4 ), 4.28(s, 5H, C 5 h 5 ), 4.01 (q, 2H, -OCH 2 ), 1.69 (s, 3H, COCH 3 ), 1.30(t, 3H, CH 2 CH 3 );
[0060] 13 C-NMR: 192.3, 158.2, 140.4, 135.0, 129.5, 128.5, 122.5, 43.6, 22.8, 13.8.
Embodiment 2
[0062] Weigh 0.0012mol ethyl acetoacetate, 0.0012mol anhydrous K 2 CO 3 Place in a mortar and mix quickly and evenly, then add 0.001mol 1-ferrocenyl-3-(p-chlorophenyl)-propenone, mix and grind. The mixture will become viscous as the reaction proceeds. Continue to grind until the substance does not change. Use thin-layer chromatography to monitor the reaction progress. After the reaction is completed, wash with pure water several times and filter to obtain a dark red solid, which is 1-2 Ferrocenyl-3-(p-chlorophenyl)-3-(1-acetyl-1-carboxyethyl-methine)-propanone. The yield was 77%, M.p. 143°C-144°C.
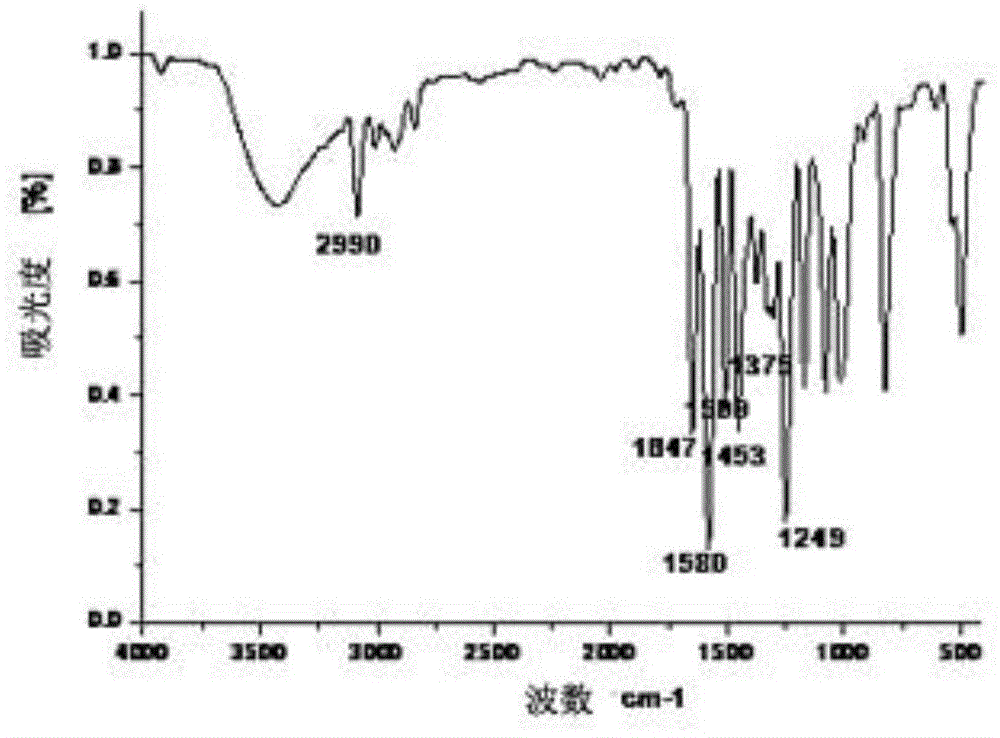
[0063] IR (KBr tablet, v / cm -1 ):2993, 1721, 1649, 1591, 1494, 1453, 1378, 1238;
[0064] 1 H-NMR: 7.16-7.63 (m, 4H, Ar-H), 4.96 (s, 2H, C 5 h 4 ), 4.66(s, 2H, C 5 h 4 ), 4.27(s, 5H, C 5 h 5 ), 4.02 (q, 2H, -OCH 2 ), 2.35(s, 3H, COCH 3 ), 1.33(t, 3H, CH 2 CH 3 );
[0065] 13 C-NMR: 192.1, 159.8, 138.9, 135.6, 133.7, 128.9, 122.9, 43.1, 23.1, 13.9.
Embodiment 3
[0067] Weigh 0.0012 mol of ethyl acetoacetate, 0.0012 mol of NaOH were placed in a mortar and quickly mixed evenly, then 0.001 mol of 1-ferrocenyl-3-(p-bromophenyl)-propenone was added, mixed and ground. The mixture will become viscous as the reaction proceeds. Continue to grind until the substance does not change. Use thin-layer chromatography to monitor the reaction progress. After the reaction is completed, wash with pure water several times and filter to obtain a dark red solid, which is 1-2 Ferrocenyl-3-(p-bromophenyl)-3-(1-acetyl-1-carboxyethyl-methine)-propanone. Yield 73%, m.p. 162°C-163°C.
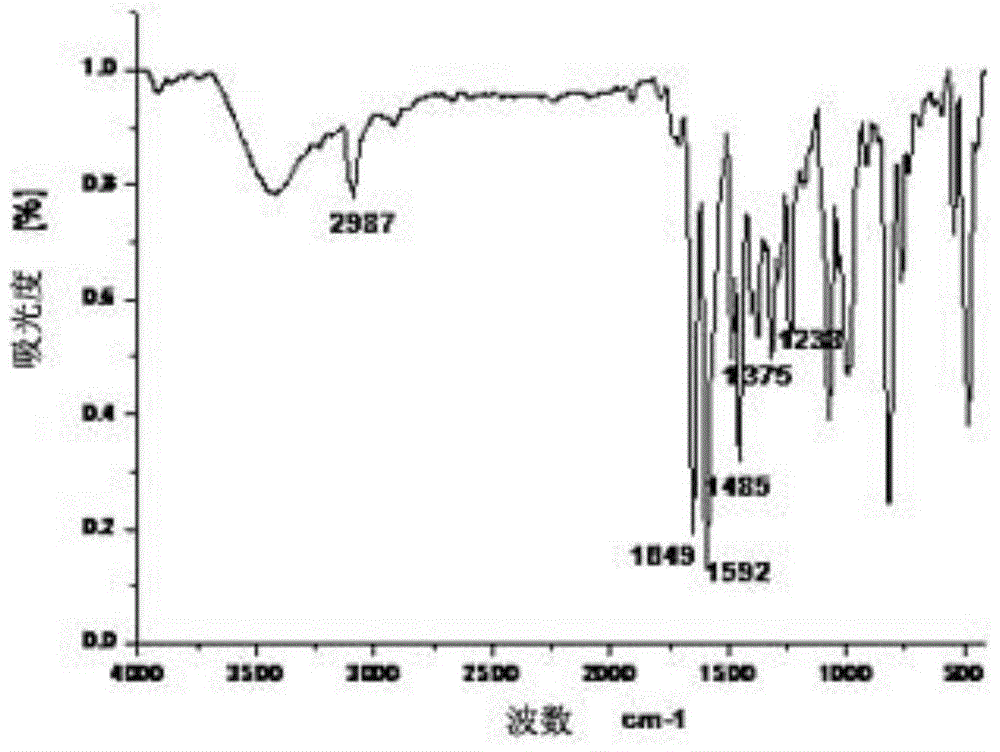
[0068] IR (KBr tablet, v / cm -1 ):2987, 1712, 1649, 1592, 1485, 1453, 1375, 1238;
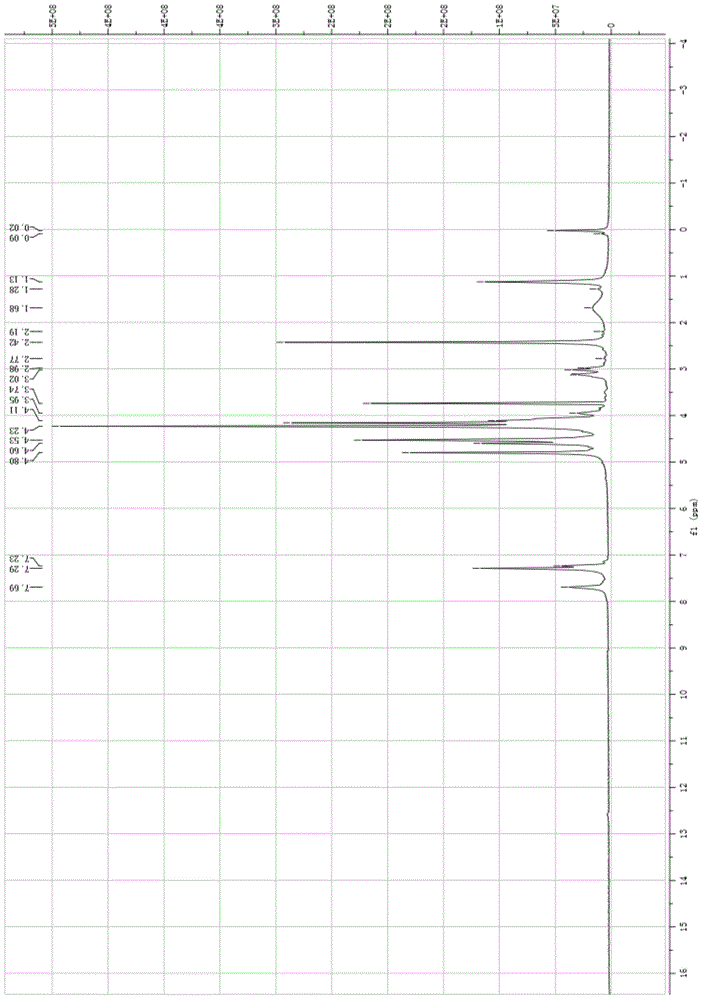
[0069] 1 H-NMR: 7.12-7.77 (m, 4H, Ar-H), 4.94 (s, 2H, C 5 h 4 ), 4.64(s, 2H, C 5 h 4 ), 4.25(s, 5H, C 5 h 5 ), 4.02 (q, 2H, -OCH 2 ), 2.23 (s, 3H, COCH 3 ), 1.14(t, 3H, CH 2 CH 3 );
[0070] 13 C-NMR: 191.2, 143.1, 138.9, 129.5, 122.0, 121.9, 119.5, 58.7, 43.0, 27.0, 13.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com