Bactrian camel-derived ApoE nano antibody as well as coding sequence and application thereof
A nanobody and sequence technology, applied in the field of biomedicine or biopharmaceuticals, to achieve high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Construction of the Nanobody Library against ApoE:
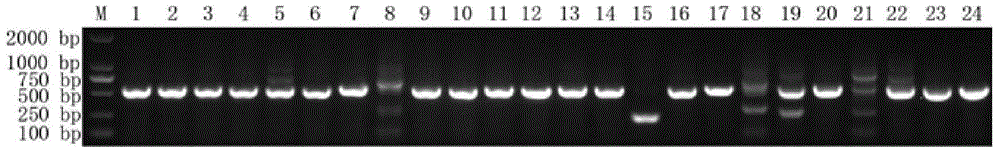
[0019] (1) First mix 1 mg ApoE with Freund's adjuvant in equal volumes, and immunize a Xinjiang Bactrian camel once a week, a total of 7 times to stimulate B cells to express antigen-specific nanobodies; (2) 7 times of immunization After the end, extract 100mL camel peripheral blood lymphocytes and extract total RNA; (3) synthesize cDNA and amplify VHH by nested PCR; (4) digest 20ug pComb3 phage display vector with restriction enzymes Pst I and Not I (supplied by Biovector) and 39ug VHH and connect the two fragments; (5) transform the ligated product into electroporation competent cell TG1, construct the ApoE nanobody library and measure the storage capacity, the storage capacity is 4.9×10 9 CFU / mL; (6) 24 clones were randomly selected, and the insertion rate of the built library was detected by colony PCR. The insertion rate was 83.3%. figure 1 The colony PCR results are shown. The DNA bands in the gel we...
Embodiment 2
[0042] Embodiment 2: Nanobody screening process against ApoE:
[0043] (1) Dissolve in 100mM NaHCO 3 , 20ug ApoE in pH 9.5 was coupled to the NUNC microtiter plate, and placed overnight at 4°C; (2) the next day, 100uL 0.1% casein was added, and blocked at room temperature for 2h; (3) after 2h, 100uL phage (5× 10 11tfu immunized camel nanobody phage display gene library), and acted at room temperature for 1h; (4) washed 5 times with PBS+0.05% Tween-20 (PBST) to wash off unbound phage; (5) washed with 100mM triethanolamine TEA ( triethylamine) to dissociate the phage that specifically binds to ApoE, and infect Escherichia coli TG1 in logarithmic phase growth, culture at 37°C for 1 h, produce and purify the phage for the next round of screening, and repeat the same screening process for 3-4 Rounds, gradually enriched.
Embodiment 3
[0044] Embodiment 3: use the enzyme-linked immunosorbent method (ELISA) of phage to screen specificity single positive clone:
[0045] (1) From the cell culture dish containing phage after the above 3-4 rounds of screening, select 96 single colonies and inoculate them in TB medium containing 100ug / mL ampicillin (1L TB medium contains 2.3g potassium dihydrogen phosphate , 12.52g dipotassium hydrogen phosphate, 12g peptone, 24g yeast extract, 4mL glycerol), after growing to the logarithmic phase, add IPTG with a final concentration of 1mM, and culture overnight at 28°C. (2) The crude antibody was obtained by infiltration method, and the antibody was transferred to an antigen-coated ELISA plate, and left at room temperature for 1 hour. (3) Unbound antibodies were washed away with PBST, and mouse anti-HA tag antibody (mouse anti-HA antibody, purchased from Beijing Kangwei Century Biotechnology Co., Ltd.) was added, and left at room temperature for 1 hour. (4) Unbound antibodies w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com