Anti-Abeta42 oligomer single-chain antibody and gene for coding single-chain antibody
A single-chain antibody and oligomer technology, applied in the field of genes encoding the single-chain antibody, to achieve the effect of reducing toxicity and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
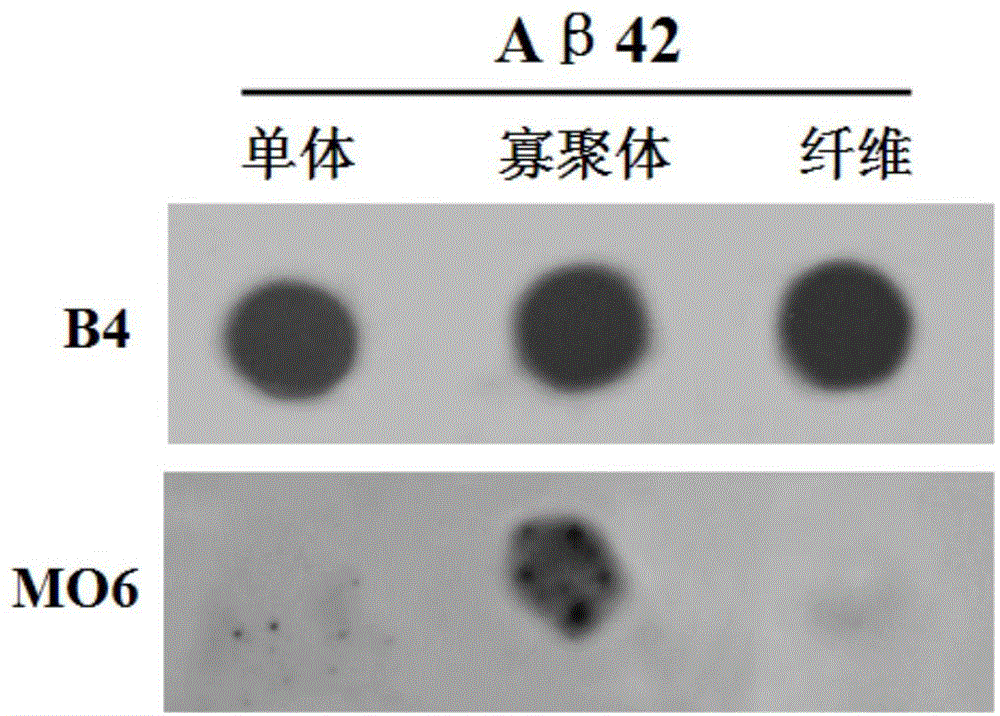
[0024] Example 1 Preparation of Aβ42 oligomers and Aβ42 fibers
[0025] Aβ42 monomer (purchased from Sigma, USA) was dissolved in ice-cold hexafluoroisopropanol (HFIP) to a concentration of 1 mg / mL, sonicated in an ice-water bath for 10 min, dried in vacuum, and frozen at -20°C. When using, first dissolve the Aβ42 monomer with dimethyl sulfoxide (DMSO) to a concentration of 1 mg / mL, then dilute Aβ42 to a concentration of 10 μM in phosphate buffer (pH 7.4, 50 mM), and the final concentration is 10 μM , respectively incubated at 37°C for 12h to form the aggregated state of Aβ42 oligomers and incubated for 3 days to form the aggregated state of mature fibers. All Aβ42 aggregation states were confirmed by electron microscopy. Due to the irreversibility of Aβ42 to form aggregates, the oligomers and fibers of Aβ42 are prepared on-site and stored at -80°C for short-term use.
Embodiment 2
[0026] Example 2 Screening of positive clones
[0027] (1) RNA extraction and reverse transcription of peripheral blood lymphocytes to synthesize cDNA
[0028] Separation and extraction of peripheral blood lymphocytes: 10 mL of human peripheral blood blood samples were collected with dipotassium ethylenediaminetetraacetic acid (EDTA-2K) anticoagulant tubes, and balanced salt buffer (D'Hanks) without calcium and magnesium ions (purchased from Bi Yuntian Biotechnology Research Institute) was diluted with 1:1 volume, and added Ficoll-diatrizoate lymphocyte separation medium (purchased from American sigma company) with the ratio of diluted blood sample and lymphocyte separation medium=2:1 (volume ratio) . Centrifuge at 2000rpm / min for 20min at room temperature. After centrifugation, the mononuclear cell layer is aspirated. The cells were washed with D'Hanks and centrifuged at 1000rpm / min for 10min to obtain peripheral blood lymphocytes.
[0029] Peripheral blood lymphocyte RNA...
Embodiment 3
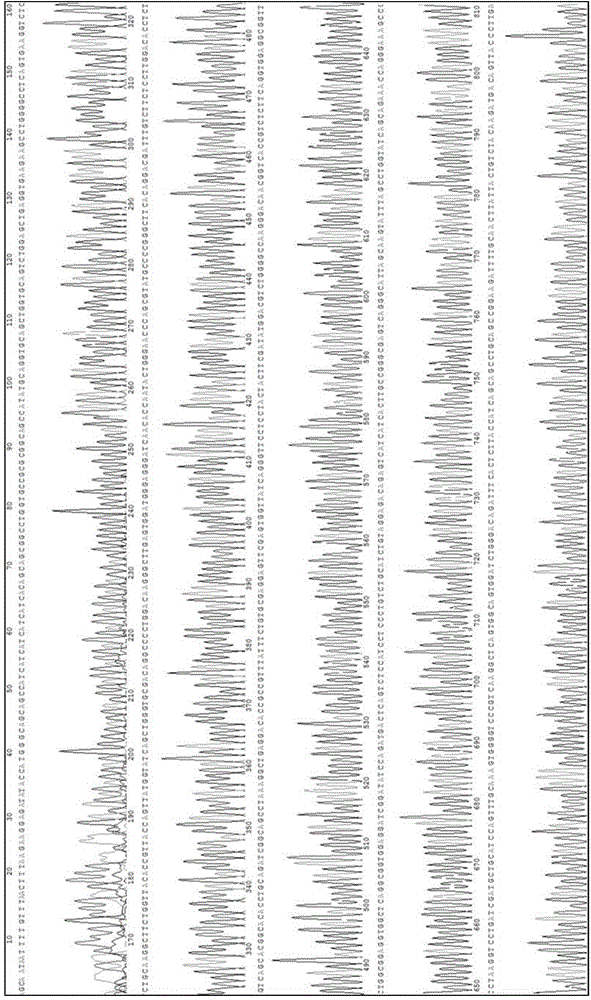
[0055] Example 3 DNA sequence determination of single chain antibody MO6 gene
[0056] The plasmid pET28a-MO6 was extracted and submitted to Shanghai Sangon Sequencing Department for sequence determination. The results are as follows: figure 1 (PET28a-MO6 carrier sequencing spectrum, wherein the gene encoding MO6 is nucleotide 38-808). The measured effective MO6 gene sequence was compared with the immunoglobulin gene sequence in GeneBank, and the results proved that the obtained MO6 clone was a DNA sequence encoding scFv, the start codon was ATG, and the stop codon was It is TGA (its effective nucleotide sequence is shown in SEQ ID NO.4), which includes the DNA sequence encoding the antibody heavy chain variable region (VH) and light chain variable region (VL), and the deduced amino acid sequence (SEQ ID NO.1, SEQ ID NO.2) have a typical antibody variable region structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com