Camptothecin nano-gel
A nano-gel, camptothecin technology, applied in the directions of non-active ingredients medical preparations, organic active ingredients, skin diseases, etc., can solve problems such as clinical application limitations, and achieve prolonging action time, reducing penetration, and increasing skin retention. amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 The preparation of camptothecin nanoparticles and gel:
[0034] Preparation of blank and drug-loaded nanoparticles:
[0035]PLGA-PAMAM-OA (PPO) nanoparticles are prepared by emulsified solvent evaporation method, get 10mgPLGA dissolved in 1.5ml methylene chloride solution obtained as the organic phase, the organic phase is added to 14.5ml 0.1% (W / V) PVA 1. In the mixed solution of 4ml 0.5% PAMAM and 1.5ml Tween 80, stir at a constant speed to form a coarse emulsion, then stir with an ultrafine homogenizer for 15min (35000rpm) to form a nanoemulsion. Evaporate the organic phase, add 100 μl 1% (W / V) sodium triphosphate (TPP) for cross-linking, then stir at 300 rpm for 2 h, suspend in phosphate buffer (pH 8.0), and incubate with oleic acid (OA) After 2 hours, the blank nanoparticle PPO was obtained, and the unmodified PLGA nanoparticle (PLGA-PAMAM) was prepared according to the above method.
[0036] The preparation method of camptothecin-loaded drug-loaded ...
Embodiment 2
[0042] Example 2 Preparation of blank and drug-loaded gel (PPO gel, CPT-PPO gel):
[0043] The preparation method of the gel is as follows: take camptothecin 0.01g and dissolve it in 40ml ethanol, ultrasonically dissolve it, add swollen hydroxypropyl methylcellulose (HPMC) drop by drop and constantly stir until fully forming a gel, Stable at room temperature for 24 hours for later use to obtain camptothecin gel (CPT gel).
[0044] The preparation method of nanogel refers to the preparation method of camptothecin gel, and HPMC is added dropwise to the blank and drug-loaded aqueous solution of nanoparticles until the gel is completely formed (PPO gel, CPT-PPO gel, PP gel, CPT-PP gel), stable at room temperature for 24 hours.
Embodiment 3
[0045] Example 3 In vitro release kinetics research:
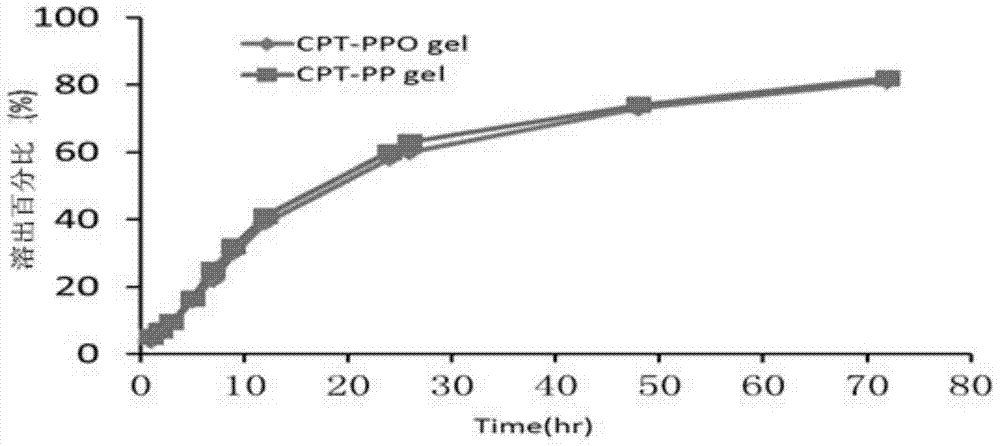
[0046] A certain amount of drug-loaded nanogels CPT-PPO and CPT-PP were put into dialysis bags respectively, placed in PBS dissolution cups with pH 7.4, the temperature was controlled at 37±1°C, and the stirring speed was 300rpm. At different times (1, 2, 4, 6, 8, 12, 22, 24, 48, 72h), take 0.5ml of dialysate, constant volume with methanol, use HPLC to measure the concentration of camptothecin, and calculate the concentration of camptothecin at different times. Dissolution percentage. The results are attached image 3 as shown..
[0047] It can be seen from the figure that the in vitro drug release of the nanogel basically followed the first-order kinetic equation, and there was no significant difference in drug release between the OA-modified drug-loaded nanogel and the unmodified drug-loaded nanogel.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
| Drug loading | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com