Synthetic method for Tildipirosin
A technology of tediloxine and synthetic chemistry, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of high toxicity of triphenylphosphine oxide mixture, inability to directly discharge or bury, pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Embodiment 1: the new synthesis of tedirol
[0106] The implementation steps of this embodiment are as follows:
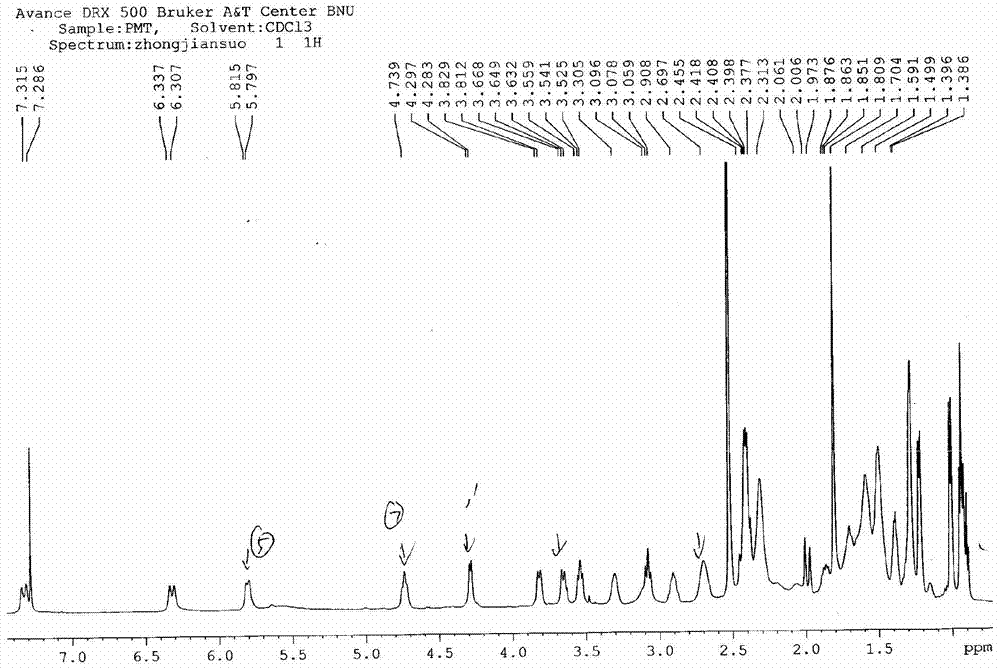
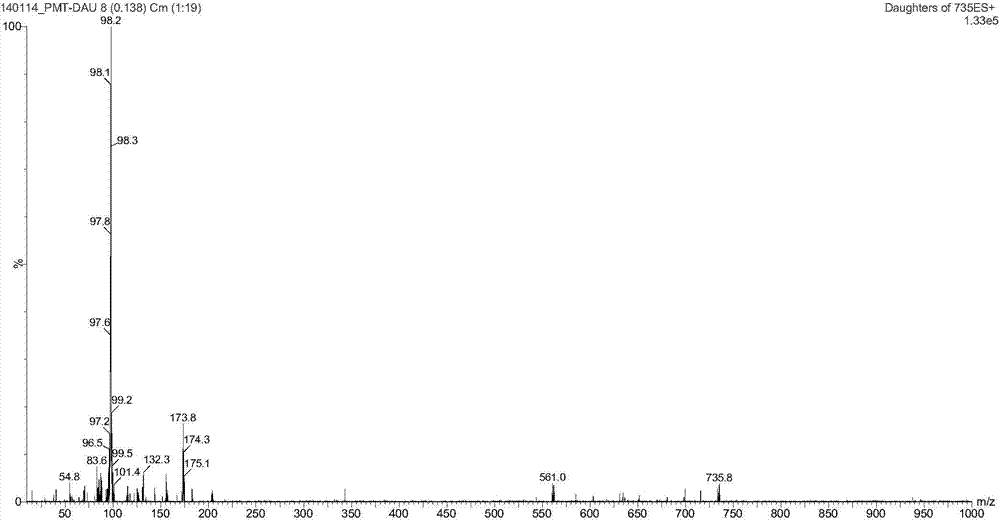
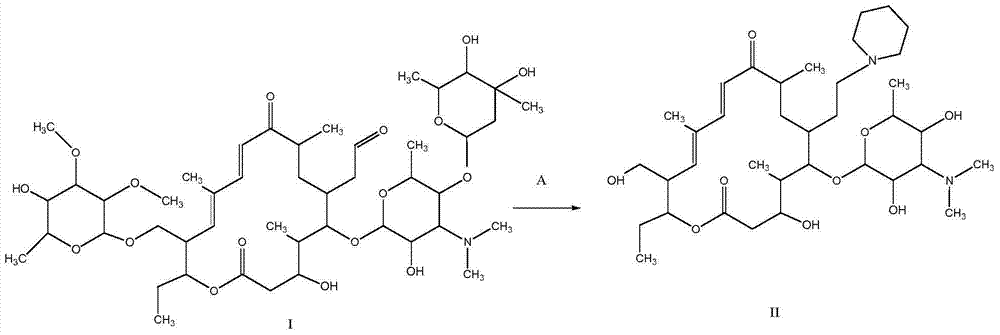
[0107] Synthesis of A, 23-hydroxyl-20-piperidinyl-5-O-mycaminosyl-tylonolide (II)
[0108] In an aqueous solution, allow 0.08 mole of tylosin (I) and 0.6 mole of sulfuric acid to react for 1.2 h under the conditions of pH 1.8 and reaction temperature 60° C., then allow the reaction mixture to cool, and then dissolve it with 25% ammonia water by volume. The pH of the reaction mixture was adjusted to pH 10, according to the volume ratio of the reaction mixture to ethyl acetate 1.0:1.0, the reaction mixture adjusted to pH was extracted 3 times with ethyl acetate under alkaline conditions, the combined extracts were then mixed with 0.10 1 mole of piperidine and 2.0 moles of hydrochloric acid were reacted at pH 1.6 and reaction temperature 70°C for 1 h; the reaction mixture was cooled and transferred to a separatory funnel, water was added, and the pH of the rea...
Embodiment 2
[0116] Embodiment 2: the new synthesis of tedrol
[0117] The implementation steps of this embodiment are as follows:
[0118] Synthesis of A, 23-hydroxyl-20-piperidinyl-5-O-mycaminosyl-tylonolide (II)
[0119] In aqueous solution, allow 0.04 mole tylosin (I) and 0.8 mole hydrochloric acid to react 0.5h under the condition of pH 1.0 and reaction temperature 90 ℃, allow reaction mixture to cool down then, use 28% ammoniacal liquor by volume again its The pH of the reaction mixture was adjusted to pH 9, according to the volume ratio of the reaction mixture to ethyl acetate 1.0:0.5, the reaction mixture adjusted to pH was extracted with ethyl acetate under alkaline conditions, the combined extracts were mixed with 0.05 mole Piperidine and 0.5 mole of sulfuric acid were reacted for 4 hours at a pH of 1.0 and a reaction temperature of 60°C; after the reaction, the reaction mixture was cooled to room temperature, transferred to a separatory funnel, water was added, and the reaction...
Embodiment 3
[0127] Embodiment 3: the new synthesis of tedrol
[0128] The implementation steps of this embodiment are as follows:
[0129] Synthesis of A, 23-hydroxyl-20-piperidinyl-5-O-mycaminosyl-tylonolide (II)
[0130] In an aqueous solution, allow 0.06 mol of tylosin (I) to react with 1.0 mol of trifluoroacetic acid at a pH of 2.5 and a reaction temperature of 95°C for 0.8h, then allow the reaction mixture to cool, and then use 28% ammonia water by volume The pH of the reaction mixture is adjusted to pH 10, according to the volume ratio of the reaction mixture to methyl acetate 1.0:0.5, the reaction mixture adjusted to pH is extracted with methyl acetate under alkaline conditions, the combined extracts are mixed with 0.12 mole Piperidine and 0.8 mole of formic acid were reacted for 3 hours under the conditions of pH 2.4 and reaction temperature 50°C; after the reaction was completed, the reaction mixture was cooled to room temperature, transferred to a separatory funnel, water was a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com