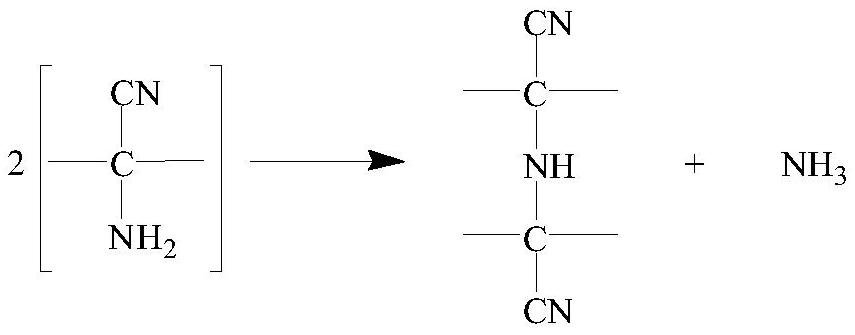
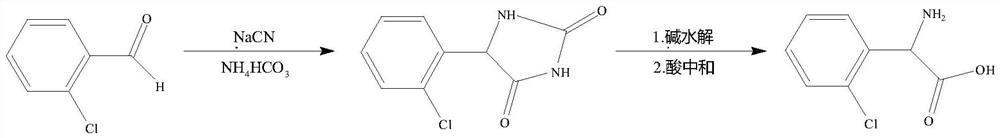
Preparation method of 2-chlorophenylglycine
A technology of o-chlorophenylglycine and o-chlorobenzaldehyde, which is applied in the field of preparation of clopidogrel drug intermediate o-chlorophenylglycine, can solve the problems of dark color and low product yield, optimize process parameters and avoid side reactions , Improve the effect of atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1) 35g o-chlorobenzaldehyde, 49.21g ammonium bicarbonate and 13.42g sodium cyanide are added in the there-necked flask of 1000mL, then 185g water and 95g methanol are added, stirring and dissolving, the insulation reaction temperature is 25-30 ℃, the insulation reaction The time is 24h, after the reaction is completed, the pH of the reaction solution is adjusted to 5.5 with 30% dilute sulfuric acid, then the methanol is removed by distillation under reduced pressure, suction filtration, and the filter cake is washed with deionized water, added to 300 g of methanol, and heated to dissolve , the insoluble impurities were filtered out by hot filtration, and then the solvent was distilled off under reduced pressure to obtain an off-white crystalline solid, which was o-chlorodihydantoin, and the HPLC content was 99.59%;
[0037] 2) 200 g of o-chlorodihydantoin was added to the sodium hydroxide solution with a mass percentage of 40%, transferred to an autoclave, stirred and he...
Embodiment 2
[0039] 1) 35g o-chlorobenzaldehyde, 59.05g ammonium bicarbonate and 14.64g sodium cyanide are added in the there-necked flask of 1000mL, then 210g water and 105g ethanol are added, stirring and dissolving, the insulation reaction temperature is 30-35 ℃, insulation reaction The time is 24h, after the reaction is completed, the pH of the reaction solution is adjusted to 6 with 30% dilute sulfuric acid, then the ethanol is removed by distillation under reduced pressure, suction filtration, the filter cake is washed with deionized water, added to ethanol, heated to dissolve, The insoluble impurities are filtered out by hot filtration, and then the solvent is distilled off under reduced pressure to obtain an off-white crystalline solid, which is o-chlorodihydantoin, and the HPLC content is 99.71%.
[0040] 2) 200 g of o-chlorodihydantoin was added to the potassium hydroxide solution with a mass percentage of 40%, transferred into the autoclave, stirred and heated to 135° C. After the ...
Embodiment 3
[0042] 1) 35g o-chlorobenzaldehyde, 68.89g ammonium bicarbonate and 15.86g sodium cyanide are added in the there-necked flask of 1000mL, then 210g water and 140g methanol are added, stirring and dissolving, the insulation reaction temperature is 30-35 ℃, insulation reaction The time is 18h, after the reaction is completed, the pH of the reaction solution is adjusted to 6.5 with 20% hydrochloric acid, then the methanol is removed by distillation under reduced pressure, suction filtration, and the filter cake is washed with deionized water, added to methanol, heated to dissolve, and heated. The insoluble impurities were filtered out, and then the solvent was distilled off under reduced pressure to obtain an off-white crystalline solid, which was o-chlorodihydantoin, and the HPLC content was 99.79%.
[0043] 2) o-chlorodihydantoin was added to 300 g of sodium hydroxide solution with a mass percentage of 30%, transferred to an autoclave, stirred and heated to 140° C. After the hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com