Preparation method of brain protein hydrolysate
A technology of cerebroprotein hydrolysate and bacterial protease, applied in the field of medicine, can solve the problems of complex process and protein denaturation, and achieve the effect of improving product content, high vitality and high peptide content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0028] Experimental Example 1 Animal Pharmacodynamics Test
[0029] Test drug: commercially available compound cerebroprotein hydrolyzate sheet (purchased from Dalian Beier Pharmaceutical Co., Ltd.), the sample that the embodiment of the present invention 5 makes, is made into the solution of required concentration with distilled water, for oral administration
[0030] use.
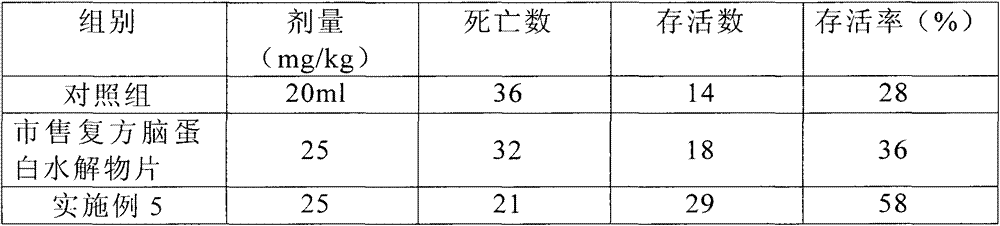
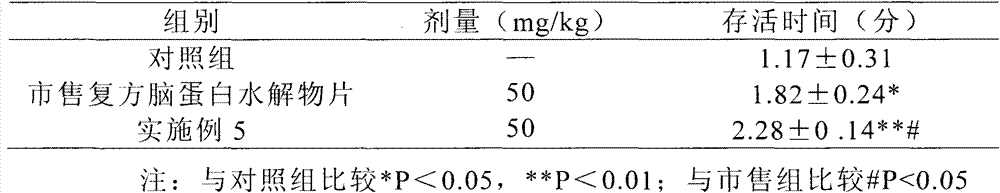
[0031] 1) Effects on the hypobaric hypoxic state of mice
[0032] Take 150 mice of 18-21g, half male and half male, and randomly divide them into 3 groups, and administer them by intragastric administration according to the drug dosage shown in Table 1. After 30 minutes, put the three groups into the vacuum chamber at the same time, airtight, and decompress Vacuum was applied, and when more than half of the mice in the control group died, observation was continued for 30 minutes after ventilation, and the number of dead animals in each group was recorded.
[0033] The results are shown in Table 1.
[0...
experiment example 2
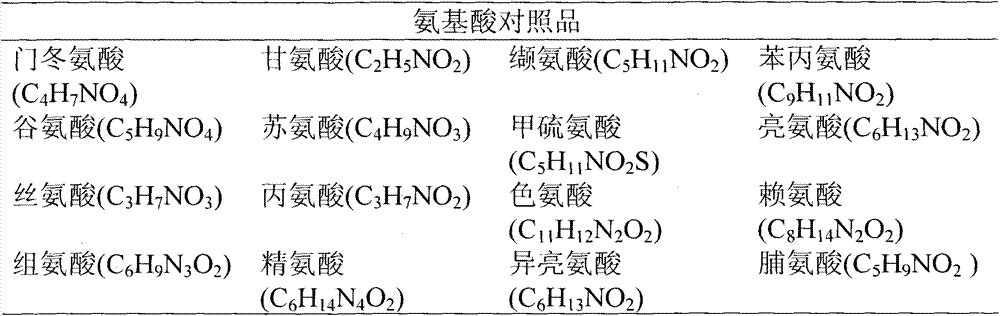
[0043] Experimental Example 2 Determination of Peptide Content in Cerebroprotein Hydrolyzate
[0044] 1. Experimental drug
[0045] Take the samples prepared according to the methods of Example 3, Example 5, Example 8 and CN200410022091.5.
[0046] 2. The method for measuring the cerebroprotein hydrolyzate content (in amino nitrogen) in the cerebroprotein hydrolyzate
[0047] Take an appropriate amount of sample (approximately equivalent to 50 mg of peptide), weigh it accurately, put it in a digestive tube, add an appropriate amount of hydrochloric acid (so that the test sample is completely submerged and does not exceed 2 / 3 of the volume of the container), fill it with nitrogen and seal it, and hydrolyze it at 110°C for 20 hours, let cool, unsealed, transfer the whole amount of the hydrolyzate to an evaporating dish, evaporate to dryness in a water bath, add water to dissolve the residue and dilute to an appropriate concentration, as the test solution for the determination o...
Embodiment 1
[0068] a) Raw material processing: Remove the capsule and blood vessels on the surface of the freshly preserved pig brain, cut it into small pieces, soak it in 0.5% sodium hydroxide solution for 48 hours, take it out and wash it, drain the water, and set aside;
[0069] b) Enzyme hydrolysis: add water to the reaction kettle containing the pig brain, add papain extracted from plants at a ratio of 10000U / g substrate, stir, and enzymolyze for 4 hours;
[0070] c) Compound enzyme hydrolysis: add compound enzyme to the product obtained in step b), stir, and enzymatically hydrolyze for 5 hours;
[0071] d) Sterilization: adjust the pH of the product obtained in step c) to 6, raise the temperature to 85° C., inactivate the enzyme for 8 minutes, add activated carbon after cooling to room temperature, stir for 4.8 hours, and then filter to obtain the filtrate;
[0072] e) concentration: the filtrate obtained in step d) is ultrafiltered with a 600Da nanofiltration membrane, and the rete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com