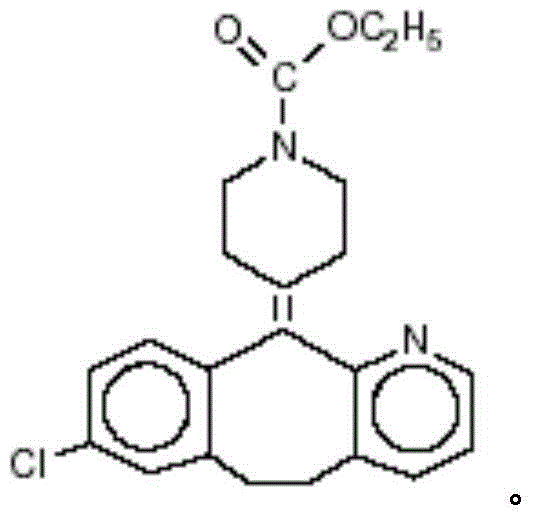
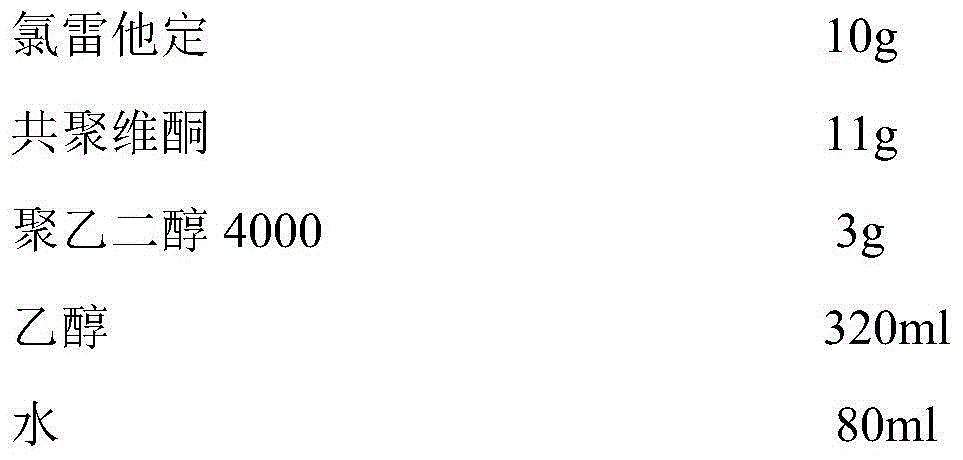Rapidly-dissolved loratadine tablets and preparation process thereof
A technology for loratadine and tablets, which is applied in the field of tablets containing loratadine and its preparation process, can solve the problems of difficult production, complex preparation process, and inability to improve bioavailability, and achieve easy operation, Simple preparation process and rapid dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Blank die
[0027] Microcrystalline Cellulose PH102 90g
[0028] Crospovidone 5g
[0030] (2) Drug-containing coating layer
[0031]
[0032] Preparation Process:
[0033] ①Weigh the microcrystalline cellulose, crospovidone, and magnesium stearate of the prescribed amount, mix them evenly, and punch them into Φ6.5mm tablets to obtain blank cores;
[0034] ②Dissolve loratadine and copovidone in ethanol, and dissolve polyethylene glycol in water at the same time, and then mix the two solutions to obtain a drug-containing coating solution;
[0035] ③Put the blank tablet core prepared in step ① into the coating pan of the high-efficiency coating machine, preheat the tablet bed to about 40°C, spray the drug-containing coating liquid prepared in step ② for coating, and dry for 30 minutes after coating is completed Left and right, that is.
Embodiment 2
[0037] (1) Blank die
[0038]
[0039] (2) Drug-containing coating layer
[0040]
[0041] Preparation Process:
[0042] ① Weigh the prescribed amount of lactose, pregelatinized starch, croscarmellose sodium, and zinc stearate, mix them evenly, and punch them into Φ7.0mm tablets to obtain blank cores;
[0043] ②Dissolve loratadine and copovidone in ethanol, and dissolve polyethylene glycol in water at the same time, and then mix the two solutions to obtain a drug-containing coating solution;
[0044] ③Put the blank tablet core prepared in step ① into the coating pan of the high-efficiency coating machine, preheat the tablet bed to about 40°C, spray the drug-containing coating liquid prepared in step ② for coating, and dry for 30 minutes after coating is completed Left and right, that is.
Embodiment 3
[0046] (1) Blank die
[0047]
[0048]
[0049] (2) Drug-containing coating layer
[0050]
[0051] Preparation Process:
[0052] ①Weigh the prescribed amount of sorbitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, polacrilin potassium, and magnesium aluminum silicate, mix them evenly, and punch them into Φ8.0mm tablets to obtain blank cores;
[0053] ②Dissolve loratadine and copovidone in ethanol, and dissolve polyethylene glycol in water at the same time, and then mix the two solutions to obtain a drug-containing coating solution;
[0054] ③Put the blank tablet core prepared in step ① into the coating pan of the high-efficiency coating machine, preheat the tablet bed to about 40°C, spray the drug-containing coating liquid prepared in step ② for coating, and dry for 30 minutes after coating is completed Left and right, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com