Cyclodextrin glucosyltransferase mutant for improving AA-2G conversion rate
A glucose-based, mutant technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of monotonous varieties, not yet reached the industrialization level, etc., and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Recombinant bacteria construction
[0023] According to the cgt gene sequence registered on NCBI (NCBI sequence number X59042.1), the cyclodextrin glucosyltransferase gene sequence cgt was synthesized by chemical synthesis. The plasmid used for the construction of E. coli was pET20b(+) with T7 promoter. The pET20b(+) plasmid and the plasmid containing the cgt gene were digested with Nco Ⅰ and Hind Ⅲ, respectively, and the digested products were recovered by tapping the rubber, and then ligated with T4 ligase, and the ligated products were transformed into E.coli JM109 competent cells, and after 37 Cultivate at ℃ for 8 hours, pick the transformant and shake culture in LB medium containing 100 mg / L ampicillin liquid, extract the plasmid, and verify by enzyme digestion to obtain the expression plasmid cgt / pET20b(+).
[0024] Transform the plasmid cgt / pET20b(+) into E.coli BL21(DE3) host bacteria, smear the LB plate containing ampicillin (100mg / L), culture at...
Embodiment 2
[0025] Embodiment 2: the preparation of mutant
[0026] (1) Single mutation
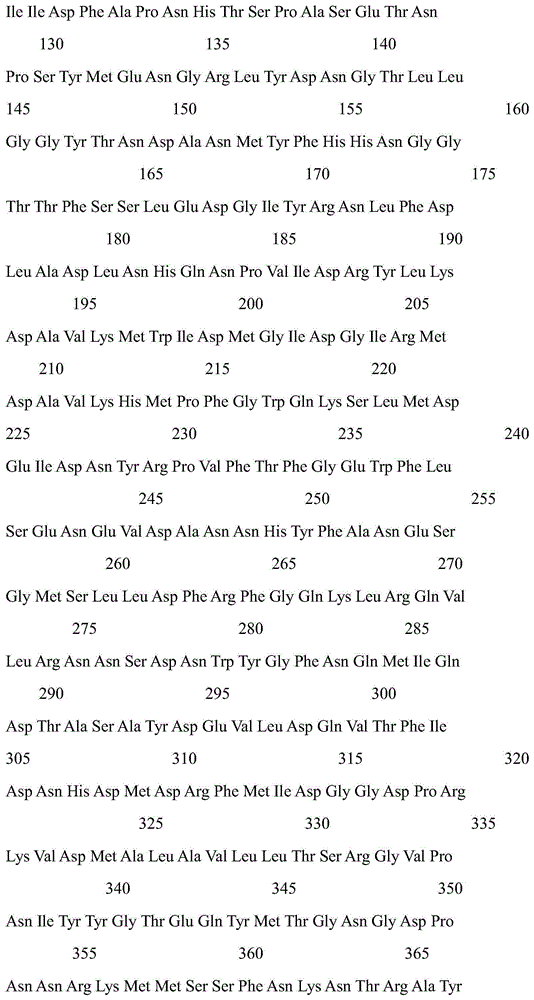
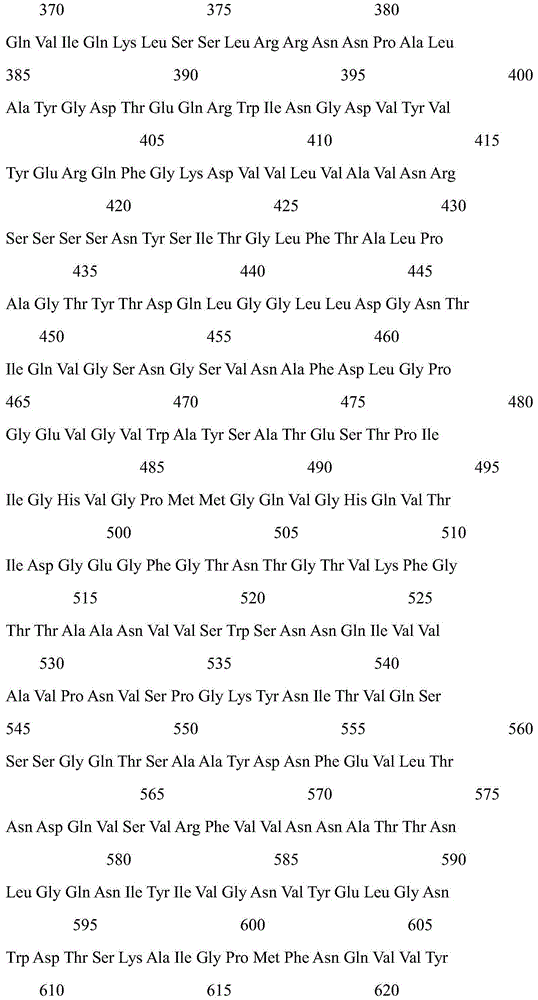
[0027] Two mutant enzymes K228R and D367S of cyclodextrin glucosyltransferase derived from B. stearothermophilus NO2:
[0028] According to the gene sequence of B. stearothermophilus NO2 cyclodextrin glucosyltransferase (NCBI sequence number X59042.1), primers for introducing K228R and D367S mutations were designed and synthesized respectively, and the cyclodextrin glucosyltransferase gene was subjected to site-directed mutation. In the DNA coding sequence, it was identified that the Lys codon at position 228 was changed to an Arg codon, and the Asp codon at position 367 was changed to a Ser codon. The mutant gene is placed in an appropriate expression vector and introduced into Bacillus subtilis, Escherichia coli or Bacillus pumilus for expression to obtain a single mutant cyclodextrin glucosyltransferase. Site-directed mutation of single mutations K228R and D367S: Using rapid PCR technology, usin...
Embodiment 3
[0048] Embodiment 3: the concentration of mutant crude enzyme liquid
[0049] Slowly add ammonium sulfate with a concentration of 26% relative to the mass fraction of the enzyme liquid to the enzyme liquid obtained in Example 2 while stirring, stir until the ammonium sulfate is dissolved, and stand at 4°C for 8-10 hours to precipitate protein. The mixture was centrifuged (8000rpm, 10min) to collect the precipitate, and the minimum volume of 50mM KH 2 PO 4 -Na 2 HPO 4 Buffer solution (pH 6.0) was redissolved, and after reconstitution, the solid matter was removed by centrifugation again, and the supernatant was collected and dialyzed to obtain a concentrated enzyme solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com