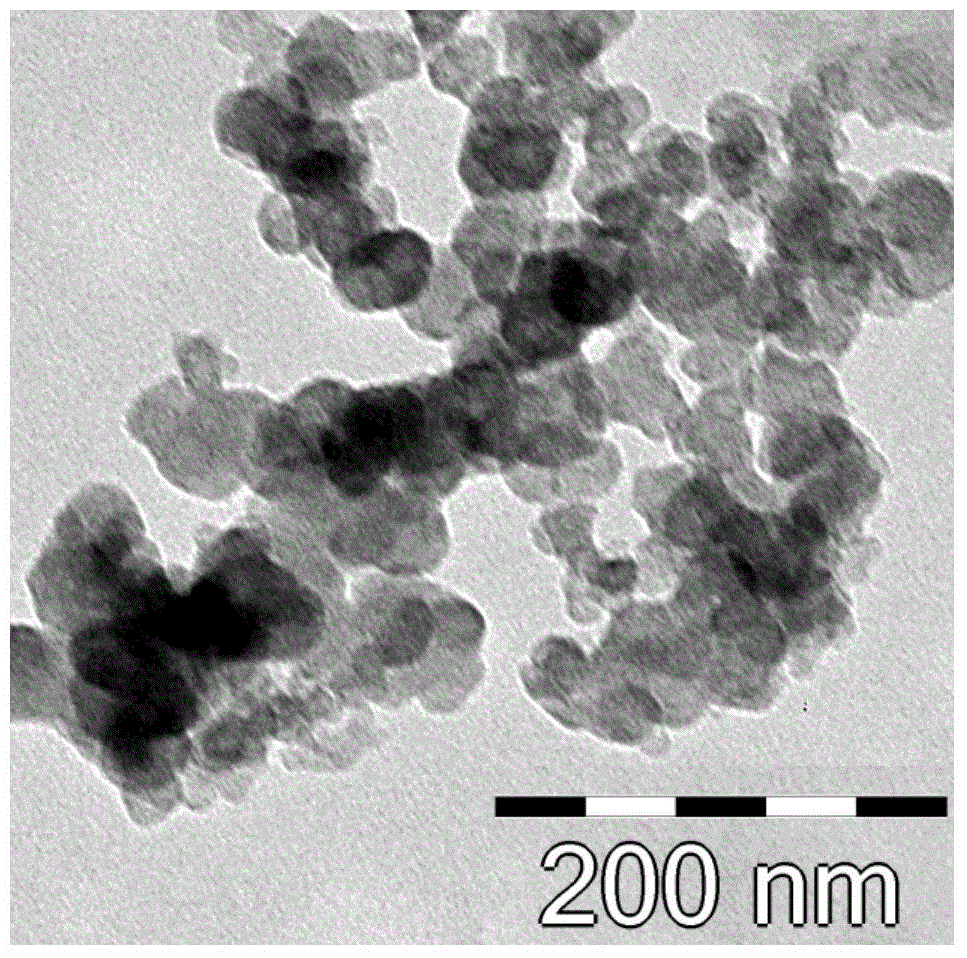
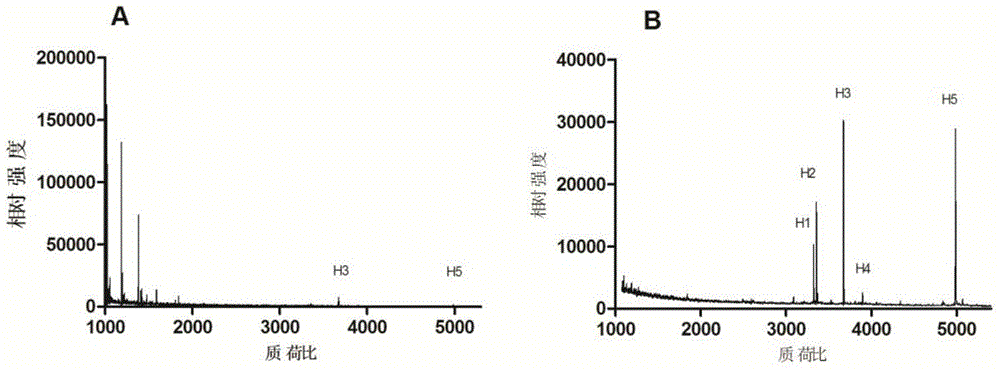
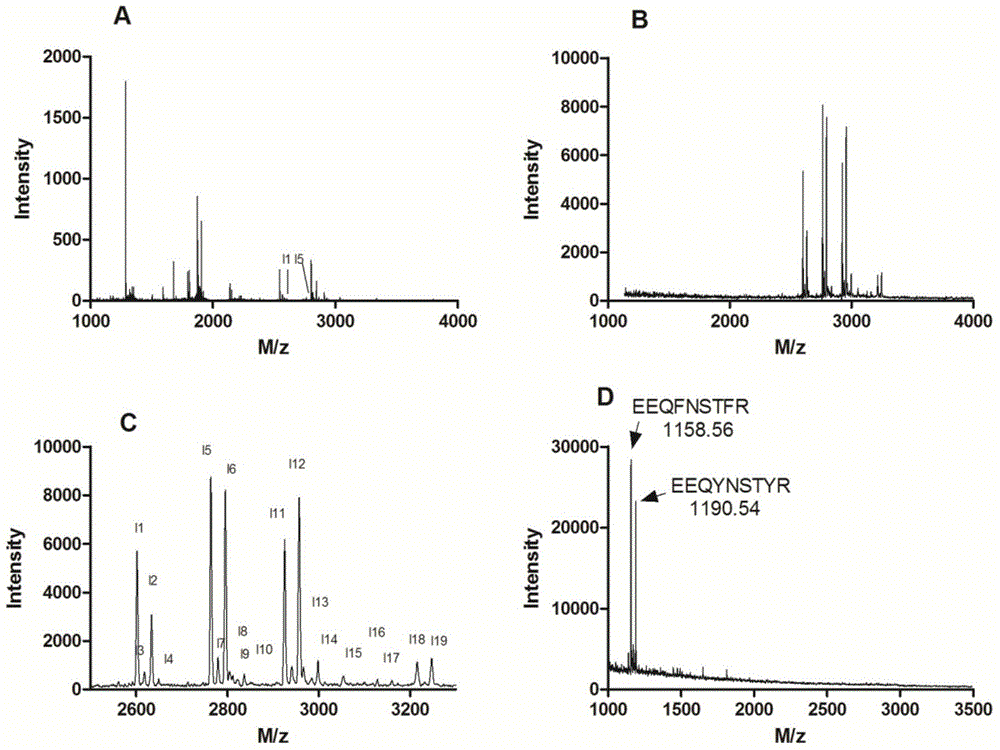
Method for enriching and purifying glycosylation peptides by nano chitosan derivative
A technology of nano-chitosan and derivatives, applied in the field of biomedical nano-materials, can solve the problem of no enrichment of glycopeptides, and achieve the effects of good biocompatibility, cheap and easy-to-obtain materials, and large specific surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Enrichment and Analysis of Horseradish Peroxidase (HRP) Glycosylated Peptides
[0029] 1) Preparation of sample solution: Add 25 μg horseradish peroxidase (Sigma) to 10 μL containing 8M urea, ethylenediaminetetraacetic acid (EDTA) and 10 mM tris(2-chloroethyl)phosphate (TCEP), shake at room temperature After 1 hour, add 40 μL of 50 mM ammonium bicarbonate solution (pH 8.2), add trypsin according to the mass ratio of trypsin (40:1) for enzymolysis reaction, and control the enzymolysis temperature at 37°C. After overnight reaction, 2% trifluoroacetic acid (TFA) was added to terminate the reaction. The obtained proteolysis solution was stored in a -80°C refrigerator for future use.
[0030] 2) Enrichment of glycosylated peptides and MALDI-TOF mass spectrometry analysis: 2 μL 1×10 -6 The mM horseradish peroxidase enzymolysis solution was dissolved in 100 μL sample solution, wherein the sample solution was an aqueous solution containing 80% acetonitrile and 5% trifluoroace...
Embodiment 2
[0033] The same method as in Example 1, wherein step 2) eluting the enriched glycosylated polypeptide with 5% to 10% ammonia solution or an aqueous solution containing 10% to 50% acetonitrile and 5% formic acid, concentrated and dried, Add 2 μL of 0.1% trifluoroacetic acid aqueous solution to dissolve, pipette 0.5 μL containing 1% HO 3 PO 4 50% acetonitrile DHB (25mg / mL) matrix solution was spotted on the target plate, 0.5 μL of the above solution containing glycosylated polypeptide was added and mixed, and then MALDI-TOF mass spectrometry analysis was performed.
Embodiment 3
[0035] The method is the same as in Example 2, wherein the mass spectrometer used is nano-LC-ESI-MS, and is carried out according to a conventional method in the art.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com