Composite carrier, pH response type compound drug-loading system and preparation method of composite carrier
A composite carrier and drug-carrying technology, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, etc., to achieve the effect of uniform size and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0121] The preparation method of composite carrier of the present invention comprises the following steps:
[0122] (a) 3-glycidyl ether trimethoxysilane is connected to the molecular chain of the carboxymethyl chitosan to obtain a compound;
[0123] (b) The composite obtained in step a) reacts with mesoporous silica, and the carboxymethyl chitosan is grafted to the surface of the mesoporous silica through 3-glycidyl ether trimethoxysilane to obtain The composite carrier.
[0124] When preparing the composite drug-loading system of the present invention, the macromolecular protein drug is loaded on the mesoporous silica, and then reacts with the complex obtained in step a) to obtain a composite drug-loading system.
[0125] In a preferred embodiment, the preparation method comprises the steps of:
[0126] (i) carboxymethyl chitosan molecule is dispersed in water, and its surface is modified with 3-glycidyl ether trimethoxysilane;
[0127] (ii) The surface-modified carboxyme...
Embodiment 1
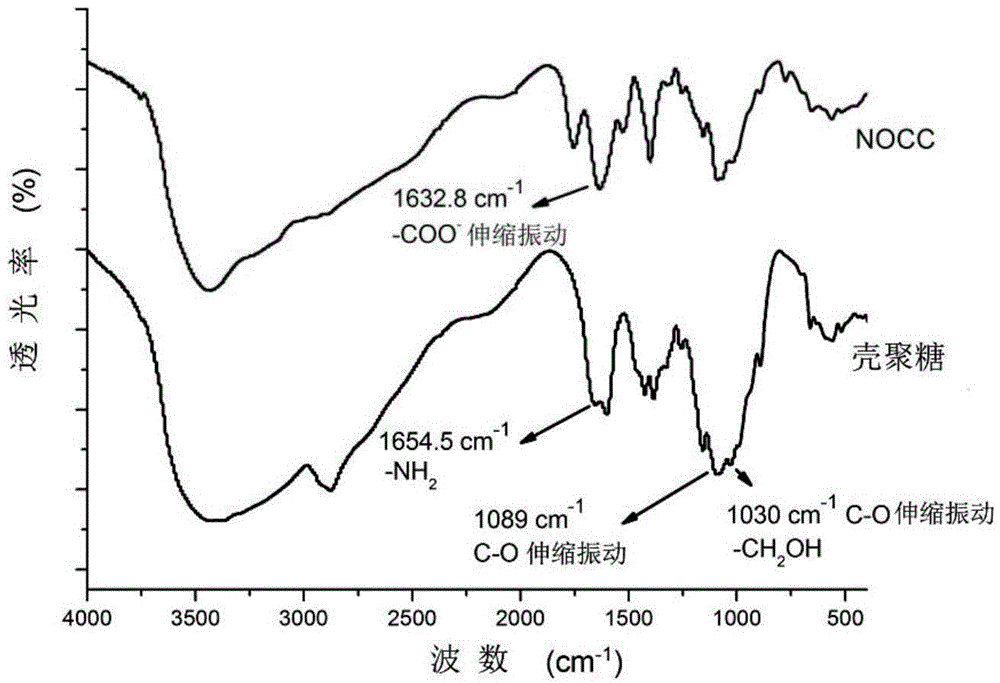
[0164] Synthesis of N,O-Carboxymethyl Chitosan (NOCC)
[0165] Weigh 10g chitosan (molecular weight 3×10 5 g / mol, degree of deacetylation 90%) powder, under stirring at room temperature, join in the 500ml three-necked flask that fills 100ml isopropanol, then 25ml concentration is that the NaOH of 10mol / L is divided into 5 parts equally, within 25min respectively Add it to the flask, and continue to stir for 30 minutes after the addition is complete.
[0166] After the stirring finishes, weigh 60g of monochloroacetic acid, divide it into 5 parts, and add a part to the flask every one minute.
[0167] After the addition, the temperature of the system was raised to 60° C., and the reaction was continued for 3 h.
[0168] The reaction was terminated, the obtained product was filtered, and the filtrate was washed with anhydrous methanol, and dried in vacuum at 60° C. to obtain a yellow solid (molecular weight 3×10 5 g / mol, degree of deacetylation 90%). Hereinafter marked as NOC...
Embodiment 2
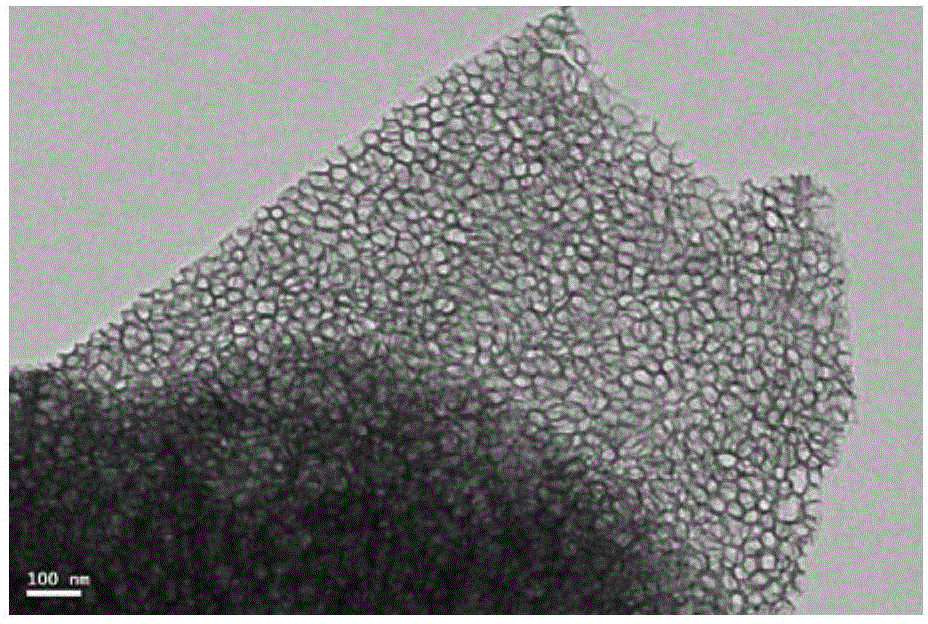
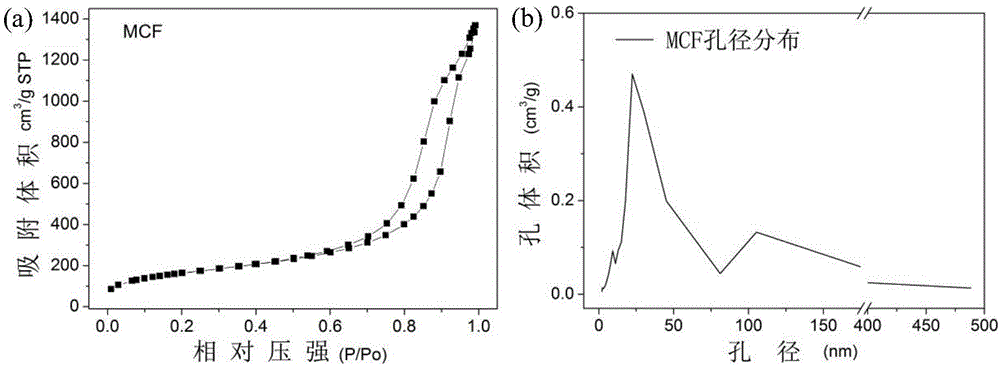
[0172] Preparation of Large Mesoporous Silica Nanoparticles 1-MCF by Sol-Gel Method
[0173] Add 10ml of concentrated hydrochloric acid with a mass fraction of 37% and 65ml of ultrapure water into the reaction vessel, mix well, and keep in a 40°C water bath.
[0174] Then, 4 g of polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer (P123) was slowly added to the mixture and stirred vigorously for about 1 h until it was completely dissolved, and the solution was in a clear state.
[0175] After the dissolution was complete, 4 g of trimethylolpropane (TMP) was added to the mixture, and kept stirring in a water bath at 40° C. for 2 h. Afterwards, 9.2 ml of tetraethyl orthosilicate (TEOS) was added dropwise and stirred vigorously, and the stirring was continued for 5 minutes after the addition was completed.
[0176] The addition of the first stage was completed, and the system was aged in a water bath at 40° C. for 20 h. Then add 46mg NH 4 F, stir gentl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com