Method for synthesizing gamma-aminobutyric acid chiral compound
A technology of chiral compounds and aminobutyric acid, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of cyanide reactions, etc., can solve the problems of expensive thiourea catalysts and lower industrial application value, and meet the reaction conditions Gentle, simple operation, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
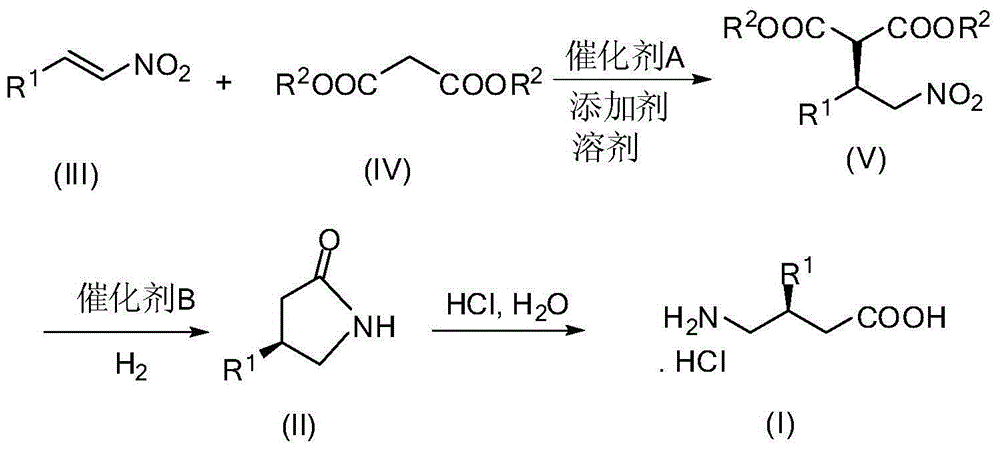
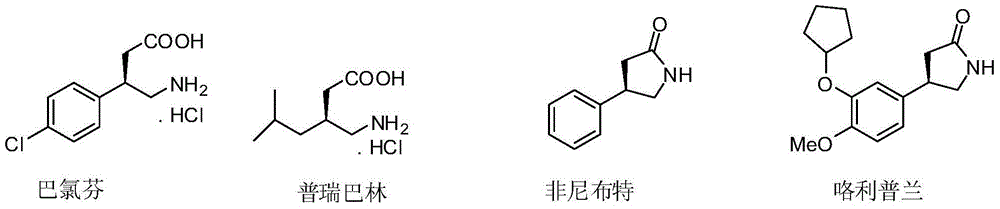
[0030] A method for synthesizing baclofen, comprising the following steps:
[0031] (1) P-chloronitrostyrene (3.66g, 20.0mmol), dimethyl malonate (7.92g, 60.0mmol), 6'-demethylquinidine (0.62g, 10mol%) and N , N-diisopropylethylamine (0.52g, 20mol%) was dissolved in THF (100mL), stirred at room temperature for 24 hours, concentrated after the reaction was completed, and purified by column chromatography (ethyl acetate / petroleum ether=1 / 10) , to obtain (R)-dimethyl 2-(1-(4-chlorophenyl)-2-nitroethyl)maleate (6.00 g, yield 95%, ee 94%).
[0032] The structural characterization data of the product are as follows:
[0033] 1 H NMR (400MHz, CDCl 3 ): δ7.31(d, J=8.4Hz, 2H), 7.18(d, J=8.4Hz, 2H), 4.91(dd, J=4.8, 12.8Hz, 1H), 4.85(dd, J=8.4, 13.6Hz, 1H), 4.23(dt, J=4.8, 9.2Hz, 1H), 3.83(d, J=9.2Hz, 1H), 3.77(s, 3H), 3.60(s, 3H); 13 C NMR (100MHz, CDCl 3 ): δ167.6, 167.0, 134.6, 134.4, 129.3, 129.2, 77.1, 54.4, 53.0, 52.9, 42.3. Chiral HPLC: Daicel chiralcel OD-H Column, Hexane / 2...
Embodiment 2
[0050] A method for synthesizing baclofen, comprising the following steps:
[0051] (1) p-chloronitrostyrene (3.66g, 20.0mmol), dimethyl malonate (7.92g, 60.0mmol), 9-O-allyl-6'-demethylquinidine ( 0.67g, 10mol%) and N,N-diisopropylethylamine (0.52g, 20mol%) were dissolved in THF (100mL), stirred at room temperature for 24 hours, concentrated after the completion of the reaction, column chromatography (ethyl acetate / Petroleum ether=1 / 10) was purified to obtain (R)-2-(1-(4-chlorobenzene)-2-nitroethyl)) dimethyl maleate (6.13g, yield 97%, ee 97%). The structural characterization data of the product are the same as in Example 1.
[0052] (2) This step is the same as in Example 1.
[0053] (3) This step is the same as in Example 1.
Embodiment 3
[0055] A method for synthesizing baclofen, comprising the following steps:
[0056] (1) P-chloronitrostyrene (3.66g, 20.0mmol), dimethyl malonate (7.92g, 60.0mmol), 9-benzoyl-6'-demethylquinidine (0.82g , 2.0mmol) and 4-dimethylaminopyridine (0.26g, 2.0mmol) were dissolved in THF (100mL), stirred at room temperature for 24 hours, after the reaction was completed and concentrated, column chromatography (ethyl acetate / petroleum ether=1 / 10) Purification to obtain (R)-2-(1-(4-chlorobenzene)-2-nitroethyl) dimethyl maleate (5.87 g, yield 93%, ee 88%). The structural characterization data of the product are the same as in Example 1.
[0057] (2) This step is the same as in Example 1.
[0058] (3) This step is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com