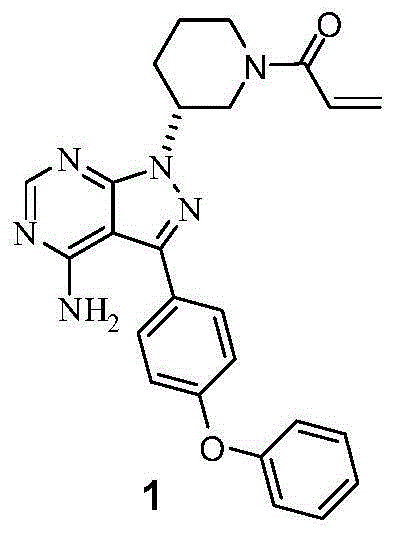
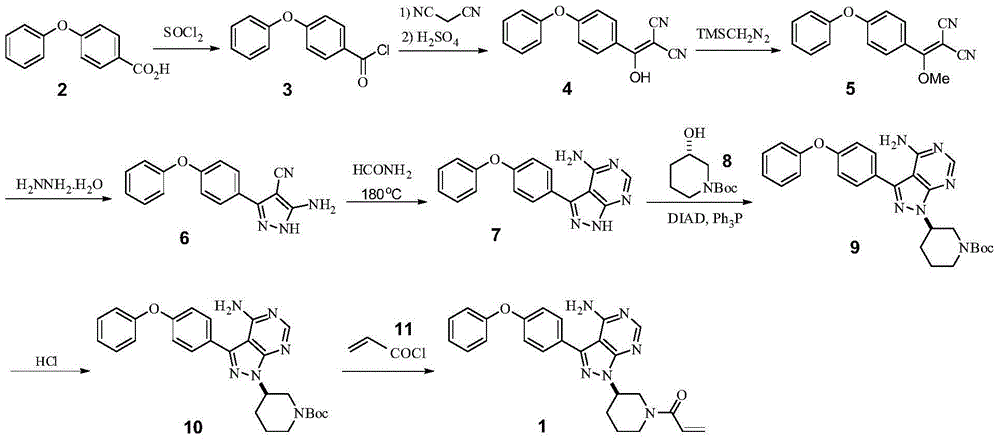
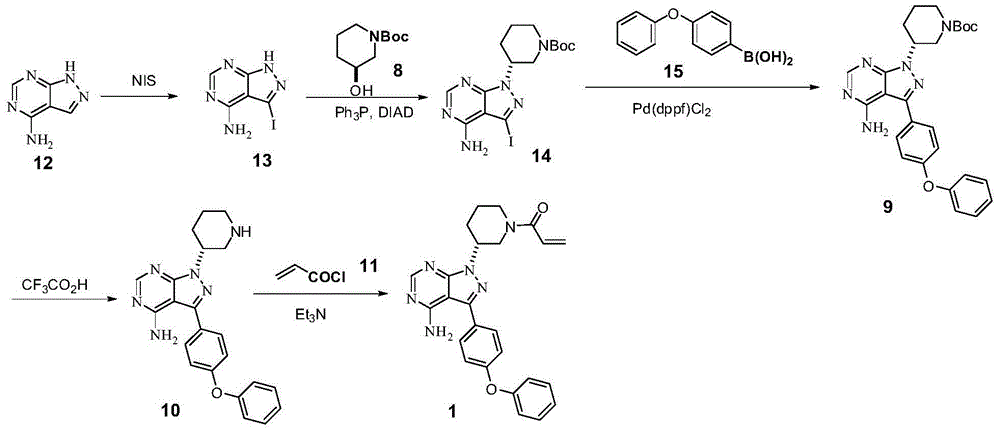
Synthesis method of ibrutinib
A technology of ibrutinib and its synthetic method, which is applied in the field of ibrutinib, can solve the problems of complex reaction system, many by-products, difficulties in industrial scale-up production, etc., and achieve the effect of short process route and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The synthesis of embodiment 1 intermediate (13)
[0047] Add 1H-pyrazolo[3,4-d]pyrimidin-4-amine (12) (13.5g, 0.1mol), N-iodosuccinimide (NIS) (25.8g, 0.15mol) to 250ml In the three-neck flask, DMF (100 mL) was added, the temperature was raised to 50° C., and then reacted at 50° C. for 18 h (TLC detection confirmed the disappearance of the raw materials). The temperature was lowered to 0°C for crystallization, and intermediate (13) (yellow solid, 20.9 g, yield 80%) was obtained after filtration and drying.
[0048] 1 H-NMR (DMSO-d6, 400 MHz, δppm): 8.17 (s, 1H).
[0049] Synthesis of intermediate (14)
[0050]
Embodiment 2
[0051] The synthesis of embodiment 2 intermediate (14)
[0052] Add intermediate (13) (10g, 0.038mol), piperidinol (8) (17g, 0.085mol), triphenylphosphine (20g, 0.076mol) into a 250ml three-necked flask, add THF (150mL), and cool to At 0°C, a mixture of DIAD (15.2 g, 0.076 mol) and THF (50 mL) was added dropwise. After the dropwise addition was completed, the mixture was slowly raised to room temperature and stirred for 20 h (TLC confirmed the disappearance of the starting material). After the reaction was completed, the reaction solution was evaporated to dryness, added MTBE (100mL) and stirred, cooled to 0°C, continued to stir for 2h after a large amount of solids were precipitated, filtered with suction, and dried to obtain intermediate (14) (12.6g, yield 75%).
[0053] 1 H-NMR (DMSO-d6, 400MHz, δppm): 1.35 (s, 9H), 1.56-1.85 (m, 2H), 1.96-2.16 (m, 2H), 2.63-2.78 (m, 2H), 2.25-2.35 (m,1H), 3.02-3.14(m,2H), 3.19-3.24(m,2H), 4.76(m,1H), 8.22(s,1H).
[0054] Synthesis of ...
Embodiment 3
[0056] The synthesis of embodiment 3 intermediate (9)
[0057] 4-Bromodiphenyl ether (X=Br) (3.74g, 15mmol) was dissolved in 1,4-dioxane (50ml), and pinacol diborate was added (4.52g, 18mmol), potassium acetate (1.78g, 18mmol). Then, the catalyst [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride [Pd(dppf) 2 Cl 2 ] (1.5mmol, 1.11g). Under stirring, it was heated to 100° C., and reacted for 5 h (the disappearance of the raw material was detected by TLC). Then, intermediate (14) (4.44 g, 10 mmol) was added, and the reaction was maintained at 100° C. for 22 h (TLC confirmed the disappearance of starting material 14). Then, after distilling off the organic solvent, intermediate (9) (yellow solid, 3.41 g, yield 70%, chemical purity and optical purity >=99%) was obtained.
[0058] 1 H-NMR (DMSO-d6, 400MHz, δppm): 1.33 (s, 9H), 1.56-1.82 (m, 2H), 1.94-2.16 (m, 2H), 2.60-2.72 (m, 2H), 2.20-2.30 (m,1H),3.00-3.12(m,2H),3.15-3.20(m,2H),4.74(m,1H),7.15(m,5H),7.40(t,J=8.2Hz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com