Preparation method of ibrutinib
A technology of ibrutinib and phenoxyphenyl, which is applied in the field of bulk drug preparation, can solve problems such as unfavorable industrialized production, lower product purity, lower yield, etc., and achieves the advantages of promoting development, environmental protection and economy, and easy availability of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
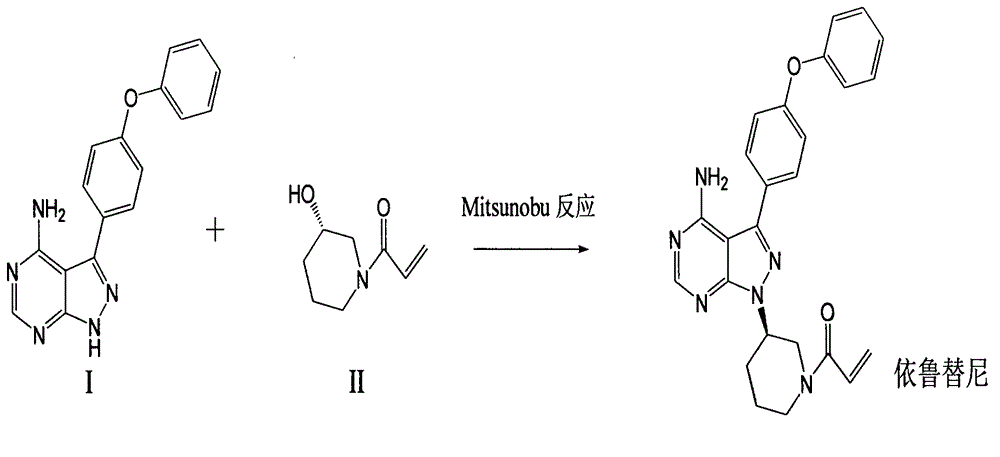
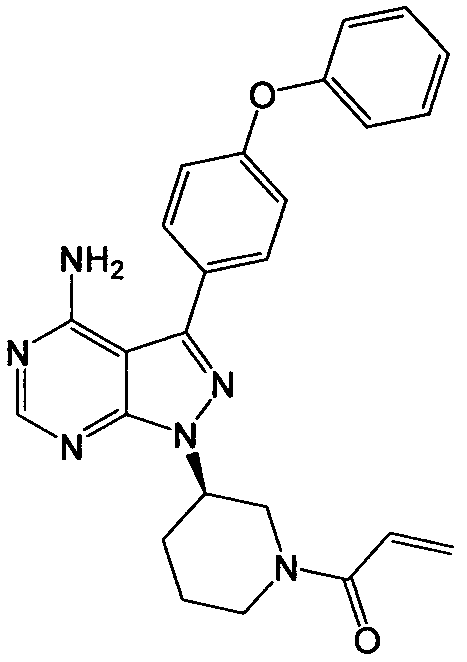
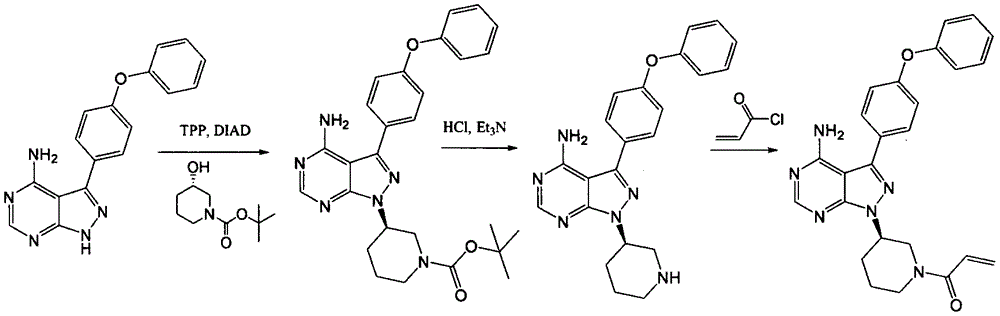
[0028] Under nitrogen atmosphere, take 30.3g 4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine and 78.6g triphenylphosphine into 1000mL tetrahydrofuran, Add 31gl-[3(S)-hydroxyl-1-piperidinyl]-2-propene-1-one to the mixture, then add dropwise 60.6g diisopropyl azodicarboxylate (DIAD), and the reaction solution is at room temperature Stir overnight. After the reaction, the reaction solution was concentrated under reduced pressure by a rotary evaporator, dissolved in dichloromethane, washed with saturated sodium chloride solution, dried, filtered, and the filtrate was concentrated and recrystallized with isopropanol to obtain an off-white solid, ibrutinib 33.2 g, yield 75.4%.
Embodiment 2
[0030] Take 10.1g of 4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine and 17.5g of polymer supported triphenylphosphine into 200mL THF, Add 10.4g (1-[3(S)-hydroxyl-1-piperidinyl]-2-propene-1-one) to the mixture, then add 13.4g diethyl azodicarboxylate dropwise, and the reaction solution is heated at 30°C After the reaction was completed, the reaction system was filtered, the filtrate was concentrated under reduced pressure by a rotary evaporator, dissolved in ethyl acetate, washed with saturated sodium chloride solution, dried, filtered, and the filtrate was concentrated and recrystallized by ethanol to obtain Irutidine Nitride 7.1g, yield 48.3%.
Embodiment 3
[0032] Add 4.4g of triphenylphosphine to 100mL of dichloromethane, add 6.8g of diphenyl azodicarboxylate dropwise, stir for half an hour, then add 5.1g of 4-amino-3-(4-phenoxyphenyl)- 1H-pyrazolo[3,4-d]pyrimidine and 3.9g (1-[3(S)-hydroxyl-1-piperidinyl]-2-propene-1-one, then the reaction solution was stirred at 0°C Overnight, the reaction solution was washed with saturated sodium chloride solution, dried, filtered, and the filtrate was concentrated and then recrystallized with isopropanol to obtain 2.7 g of ibrutinib with a yield of 37.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com