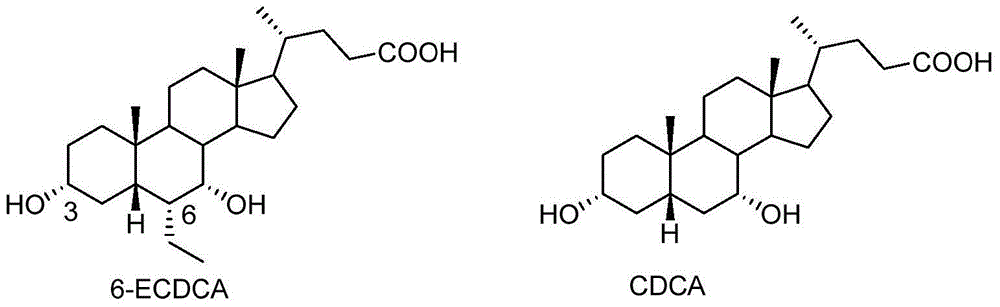
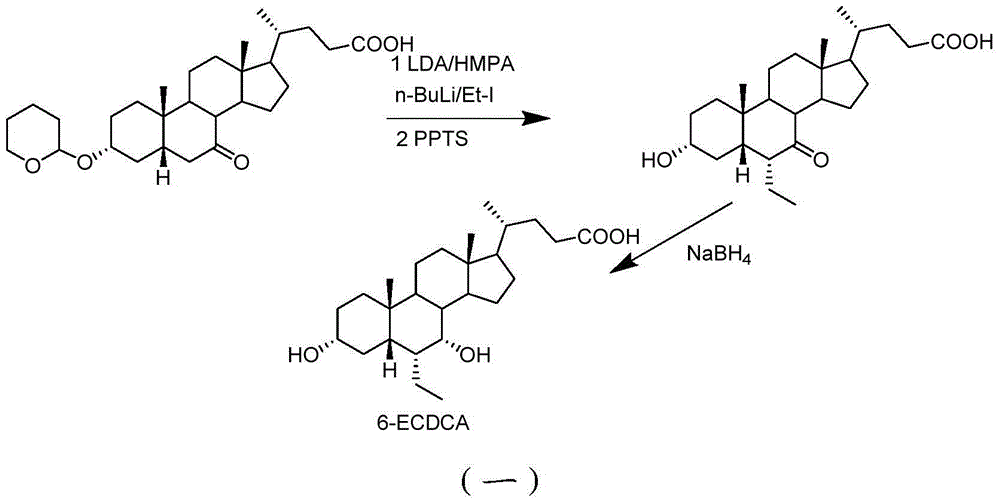
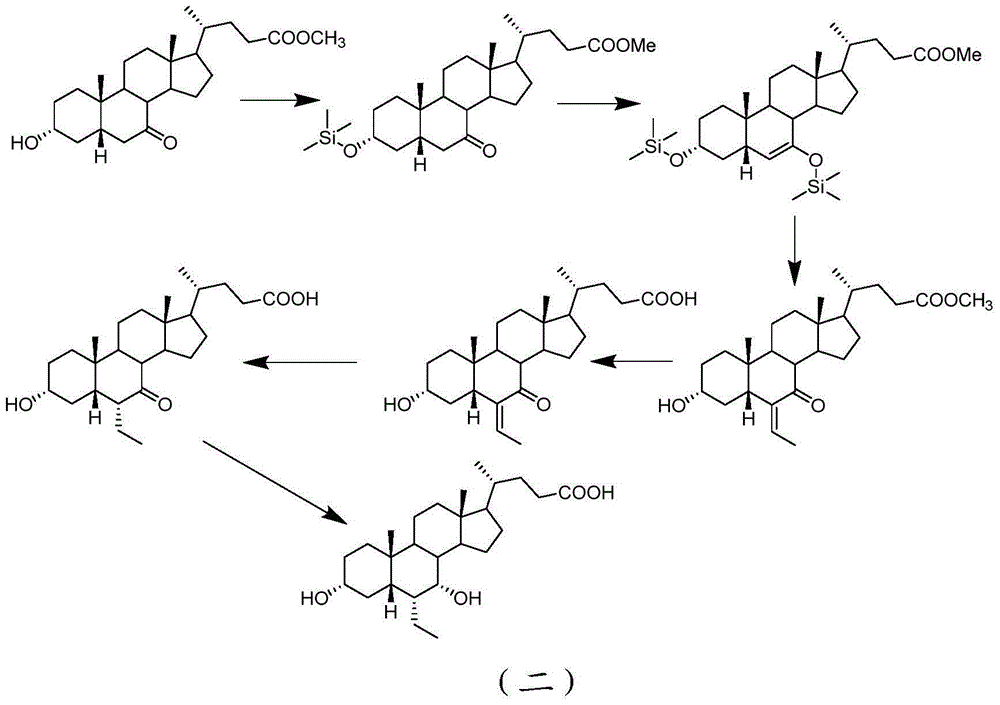
Preparation method for 5 beta-3 alpha, 7 alpha-dihydroxy-6 alpha-ethyl-cholanic acid
A technology of cholanic acid and dihydroxy, which is applied in the field of drug preparation, can solve the problems of cholic acid without high-purity raw materials and inconvenient operation, and achieve the effect of being suitable for industrial production, high product yield, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of 6-ECDCA:
[0042] Step 1: Dissolve methyl 5β-3α-ethoxycarbonyloxy-7-carbonylcholanate in tetrahydrofuran with lithium diisopropylamide, cool down to -30°C, add tert-butyldimethyl chloride Silane, stirred, gradually raised to room temperature, added saturated sodium bicarbonate solution and dichloromethane, separated the dichloromethane layer, washed twice with saturated sodium bicarbonate solution, dried with anhydrous sodium sulfate, filtered off anhydrous sodium sulfate , dichloromethane was recovered under reduced pressure to dryness to obtain a residue. Add dichloromethane to the residue, lower the temperature to below -30°C, add acetal, add anhydrous tin tetrachloride dropwise, stir, gradually rise to room temperature and stir, add water to stir, separate the dichloromethane layer, and water After washing twice, dichloromethane was recovered under reduced pressure to obtain methyl 5β-3α-ethoxycarbonyloxy-6-ethylene-7-carbonylcholanate, whic...
Embodiment 2
[0047] A preparation method of 6-ECDCA:
[0048] Step 1: Dissolve 20 grams of methyl 5β-3α-ethoxycarbonyloxy-7-carbonylcholanate in 200 mL of tetrahydrofuran, cool to -20°C, add 65 mmoles of lithium diisopropylamide (LDA), Stir for 10 minutes, add 6.5 grams of bromotrimethylsilane, stir for 1 hour, gradually rise to room temperature, add 200 mL of saturated sodium bicarbonate solution and 400 mL of dichloromethane, separate the dichloromethane layer, and wash with 150 mL of saturated sodium bicarbonate solution Twice, dry with anhydrous sodium sulfate, filter off anhydrous sodium sulfate, recover dichloromethane under reduced pressure to dryness, and obtain a residue. Add 250 mL of dichloromethane to the residue, lower the temperature to below -20°C, add 7.5 g of acetal, dropwise add 50 g of magnesium bromide ether, stir for 4 hours, gradually rise to room temperature and stir for 2 hours, add 200 mL of water and stir for 10 Minutes, the dichloromethane layer was taken separa...
Embodiment 3
[0057] A preparation method of 6-ECDCA:
[0058] Step 1: Dissolve 10 grams of methyl 5β-3α-ethoxycarbonyloxy-7-carbonylcholanate in 100 mL of tetrahydrofuran, cool down to -60°C, add 35 mmoles of lithium diisopropylamide (LDA), Stir for 10 minutes, add bromotrimethylsilane 4mL, stir for 1 hour, gradually rise to room temperature, add 200mL saturated sodium bicarbonate solution, 300mL dichloromethane, separate the dichloromethane layer, wash with 150mL sodium bicarbonate saturated solution for 2 Once, dry with anhydrous sodium sulfate, filter off anhydrous sodium sulfate, recover dichloromethane under reduced pressure to dryness, and obtain a residue. Add 150 mL of dichloromethane to the residue, lower the temperature to below -60°C, add 2 g of acetaldehyde, 30 mL of boron trifluoride ether, stir for 1 hour, gradually increase to room temperature and stir for 2 hours, add 100 mL of water and stir for 5 minutes, divide Take the dichloromethane layer, wash it twice with 100mL wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com